Abstract
In generic drug development, comparative pharmacokinetic (PK) studies are conducted to assess equivalence in pharmacokinetics and safety profiles between test and reference formulations. However, there is no established quantitative approach available for safety assessment. This study aimed to propose a method for drug safety evaluation in generic drug development, as assessed by drug influence on blood pressure and heart rate change. Data were taken from a randomized, open label, 2-way cross-over comparative PK study for megestrol conducted in 39 healthy male volunteers. Vital signs of systolic blood pressure (SBP), diastolic blood pressure (DBP) and heart rate (HR) were measured at 0 (pre-dose), 4, 8, 12, 24, 48, 72, 96 and 120 hours after the dose. Safety parameters used in the analysis were area under vital sign change versus time curve to the last measured time (AUVlast) and maximum vital sign change (Vmax). Considering highly variable nature of vital signs, the scaled bioequivalence approach developed by US FDA was adopted as a decision rule for safety evaluation between formulations. With the FDA scaled approach, 90% confidence intervals of geometric mean ratio for DBP, 0.7969∼1.0377 for Vmax and 0.7304∼1.0660 for AUVlast, were both included in the equivalence ranges of 0.7694∼1.2997 and 0.6815∼1.4674, respectively, and similarly, those for HR were included in their respective scaled equivalence limits, while SBP satisfied the conventional equivalence criterion of 0.8–1.25. These results illustrate the feasibility of applying the suggested approach in cardiovascular safety evaluation in a generic drug.
References
1. An analysis of trends in the development of generic medicines in Korea, 2014. Ministry of Food and Drug Safety. http://www.mfds.go.kr/jsp/common/download.jsp?fileinfo=S∗1∗2.5. %20%C0%D3%BB%F3 %C1%A6%B5%B5%B0%FA.hwp∗de3381f2cbc542a3f284ecd83136d51d ∗hwp∗/files/upload/1/TB_O_NOTIFY/26435/de3381f2cbc542a3f284ecd 83136d51d∗3744256∗2015: 02: 05%2009: 29: 38 Accessed 4th, AUG. 2015.
2. An analysis of trends in clinical research approvals in Korea, 2014. Ministry of Food and Drug Safety. http://www.mfds.go.kr/jsp/common/download.jsp. ?fileinfo=S∗1∗1.30_%C0%D3%BB%F3%C1%A6%B5%B5%B0%FA.hwp∗ cf0799dc431d4c439f950d1ed3cd9190∗hwp∗/files/upload/1/TB_O_NOTI-FY/26363/cf0799dc431d4c439f950d1ed3cd9190∗790016 ∗2015: 01: 30%2009: 27: 53 Accessed 4th, AUG. 2015.
3. Guideline addresses basic requirements for bioequivalence studies submitted for generic products. http://www.ich.org/fileadmin/Public_Web_Site/ABOUT_ICH/Organisation/GCC/Topics_under_Harmonisation/Bioequiva-lence.pdf. Accessed 1st, APR. 2015.
4. Guidance for Industry Bioavailability and Bioequivalence Studies for Orally Administered Drug Products –General Considerations. U.S. Department of Health and Human Services Food and Drug Administration, Center for Drug Evaluation and Research (CDER), Center for Biologics Evaluation and Research (CBER).http://www.fda.gov/downloads/drugs/guidanceco-mplianceregulatoryinformation/guidances/ucm070124.pdf. Accessed 4th, AUG. 2015.
5. GUIDELINE ON THE INVESTIGATION OF BIOEQUIVALENCE. European Medicines Agency. http://www.ema.europa.eu/ema/pages/includes/do-cument/open_document.jsp?webContentId=WC500070039. Accessed 4th, AUG. 2015.
6. Karalis V, Symillides M, Macheras P. Bioequivalence of highly variable drugs: a comparison of the newly proposed regulatory approaches by FDA and EMA. Pharm Res. 2012; 29:1066–1077.

7. Deschamps B, Musaji N, Gillespie JA. Food effect on the bioavailability of two distinct formulations of megestrol acetate oral suspension. Int J Nanomedicine. 2009; 4:185–192.
8. Chu H-M, EI . E. The Confluence of Pharmacometric Knowledge Discovery and Creation in the Characterization of Drug Safety. In Ette EI & Williams PJ (Eds). Pharmacometrics: The Science of Quantitative Pharmacology. A John Wiley & Sons. 2007. 1175–1196.
9. Baek I-h, Seong, Soo-hyeon, Kwon, Kwang-il Bioequivalence Approaches for Highly Variable Drugs: Issue and Solution. Korean Journal of Clinical Pharmacy. 2009; 19:50–60.
10. Requirements for Bioequivalence Test. Ministry of Food and Drug Safety. http://www.law.go.kr/. %ED%96%89%EC%A0%95%EA%B7%9C%EC% B9%99/%EC%9D%98%EC%95%BD%ED%92%88%EB%8F%99%EB %93%B1%EC%84%B1%EC%8B%9C%ED%97%98%EA%B8%B0%E C%A4%80 Accessed 4th, AUG. 2015.
11. Guidance for Industry Bioequivalence Studies with Pharmacokinetic Endpoints for Drugs Submitted Under an ANDA DRAFT GUIDANCE. U.S. Department of Health and Human Services Food and Drug Administration, Center for Drug Evaluation and Research (CDER), Center for Biologics Evaluation and Research (CBER).http://www.fda.gov/downloads/drugs/guidancecomplianceregulatoryinformation/guidances/ucm377465.pdf. Accessed 4th, AUG. 2015.
12. Davit B, Braddy AC, Conner DP, Yu LX. International guidelines for bioequivalence of systemically available orally administered generic drug products: a survey of similarities and differences. AAPS J. 2013; 15:974–990.

13. Cardiac Saftey Research Consortium. http://cardiac-safety.org/Accessed. 27th, MAR. 2015.
14. Critical Path Initiative. http://www.fda.gov/ScienceResearch/SpecialTop-ics/CriticalPathInitiative/default.htm. Accessed 30th, MAR. 2015.
15. Sager P, Heilbraun J, Turner JR, Gintant G, Geiger MJ, Kowey PR, et al. Assessment of drug-induced increases in blood pressure during drug development: report from the Cardiac Safety Research Consortium. Am Heart J. 2013; 165:477–488.

16. Blankfield RP. Letter to the Editor regarding "Assessment of drug-induced increases in blood pressure during drug development: report from the Cardiac Safety Research Consortium". Am Heart J. 2013; 166:e9. doi: 10. 1016/j.ahj.2013.06.008.

17. White WB, Sager PT. Response to Letter to the Editor by Blankfield regarding "Assessment of drug-induced increases in blood pressure during drug development: report from the Cardiac Safety Research Consortium." Am Heart J. 2013; 166:e11. doi: 10.1016/j.ahj.2013.06.007.
Figure 1.
Mean (SD) vital sign change versus time profiles of (A) systolic blood pressure, (B) diastolic blood pressure and (C) heart rate for reference (R) and test formulation (T).
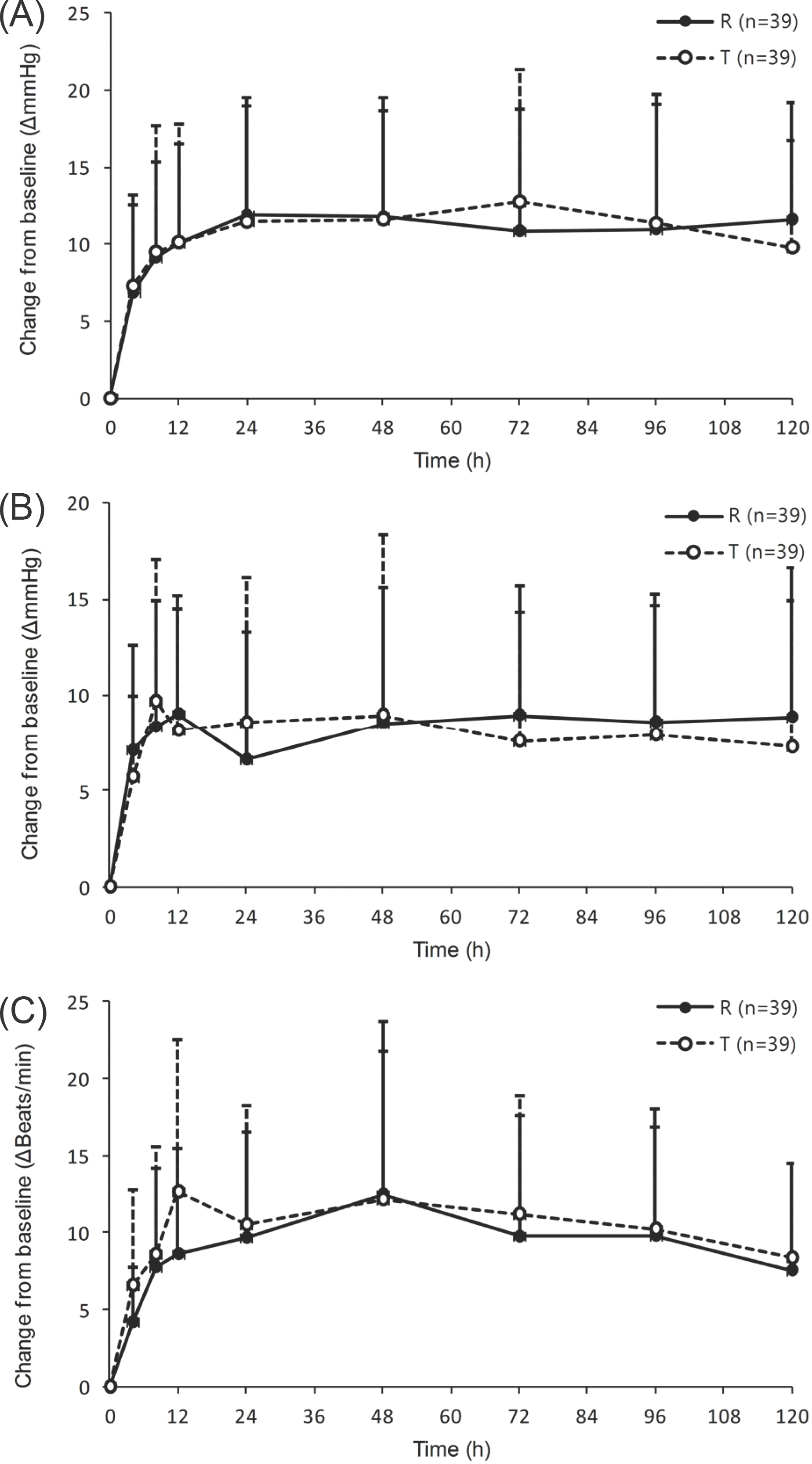
Table 1.
Demographic characteristics of the subjects
Table 2.
Scaled bioequivalence limits for highly variable drugs imposed by the FDA approach
| Within-subject CV (%) | Lower limit∗ | Upper limit∗ |
|---|---|---|
| 30 | 0.7694 | 1.2997 |
| 35 | 0.7382 | 1.3547 |
| 40 | 0.7089 | 1.4106 |
| 45 | 0.6815 | 1.4674 |
| 50 | 0.6558 | 1.5248 |
Table 3.
Comparison of safety parameters (AUVlast and Vmax) in reference and test formulations
| Parameter∗ | Geometric Mean | Geometric Mean Ratio Test/Reference | P-value+ | Residual variance | CVwR (%) | Scaled bioequivalence limit | ||
|---|---|---|---|---|---|---|---|---|
| Test (n= 39) | Reference (n= 39) | Ratio | 90% CI | |||||
| SBP | ||||||||
| Vmax | 19.65 | 19.78 | 0.9930 | 0.8804∼1.1200 | 0.9218 | 0.0992 | 31.49 | 0.7694∼1.2997 |
| AUVlast | 1201.1 | 1204.8 | 0.9978 | 0.8551∼1.1643 | 0.9809 | 0.1631 | 40.39 | 0.7089∼1.4106 |
| DBP | ||||||||
| Vmax | 15.32 | 16.85 | 0.9094 | 0.7969∼1.0377 | 0.2323 | 0.1193 | 34.53 | 0.7694∼1.2997 |
| AUVlast | 784.8 | 889.4 | 0.8824 | 0.7304∼1.0660 | 0.2714 | 0.2446 | 49.46 | 0.6815∼1.4674 |
| HR | ||||||||
| Vmax | 18.77 | 17.34 | 1.0923 | 0.9274∼1.2632 | 0.2833 | 0.1634 | 40.43 | 0.7089∼1.4106 |
| AUVlast | 1098.6 | 968.4 | 1.1335 | 0.9335∼1.3763 | 0.3932 | 0.2580 | 50.79 | 0.6558∼1.5248 |
∗ Parameter units are ΔmmHg for Vmax and ΔmmHg∗h for AUVlast for SBP and DBP, and ΔBeats/min for Vmax and ΔBeats/min∗h for AUVlast for HR.
+ P-values were obtained using WinNonlin bioequivalence test with significance level of 0.05. AUVlast: area under vital sign change versus time curve to the last measured time. Vmax: maximum vital sign change. CVwR (%): coefficient of variation for within subject variability of the reference product, which was approximated as 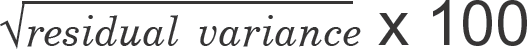 (%), in this work.
(%), in this work.




 PDF
PDF ePub
ePub Citation
Citation Print
Print


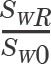 ) where SWR is standard deviation corresponding to within-subject variability of the reference product and SW0 is set at 0.25. See text for details.
) where SWR is standard deviation corresponding to within-subject variability of the reference product and SW0 is set at 0.25. See text for details. XML Download
XML Download