Abstract
Background
Pregabalin has been studied as a single or multimodal analgesic drug for postoperative pain management in different types of surgeries. We evaluated the analgesic effect of 150 mg of pregabalin in resolving post-gastrectomy pain.
Methods
Forty-four patients were randomized into two groups: a pregabalin group that received oral pregabalin (150 mg) 2 h before anesthetic induction, and a control group that received placebo tablets at the same time. Data on postoperative pain intensity (visual analog scale [VAS], at 30 min, 2 h, 4 h, and 24 h), consumption of fentanyl in patient-controlled analgesia (PCA), and the proportion of patients requiring rescue analgesics at different time intervals (0-2 h, 2-4 h, and 4-24 h) were collected during the 24 h postoperative period.
Post-surgical pain, a major complication of general surgery, has an incidence of 41-86%[1]. Adequate management of acute postoperative pain might increase patient satisfaction and reduce the risk of progression to chronic pain and associated comorbidities [2]. Specifically, control of neuropathic pain as well as inflammatory pain is considered crucial for preventing chronic postoperative pain.
Pregabalin is effective in treating neuropathic pain [3]. Recently, pregabalin has been studied as a single or multimodal analgesic drug for postoperative pain management in different surgeries [4,5]. A decrease in pain scores, superior postoperative analgesia, and opioid sparing effects have been observed during the perioperative period after preemptive and preventive analgesia using pregabalin [5,6]. However, sedation-related problems and postoperative confusion may be attributable to side effects of pregabalin [7].
We evaluated the effect of pregabalin (150mg) on post-gastrectomy pain scores, total consumption of fentanyl in patient-controlled analgesia (PCA), the proportion of patients requiring rescue analgesics, and pregabalin-related side effects in patients undergoing gastrectomy under general anesthesia.
After approval by an institutional ethics committee, written informed consent was obtained from patients. Forty-four patients aged 20-65 years, with American Society of Anesthesiologists (ASA) physical status classifications of I or II, and scheduled for open gastrectomy under general anesthesia were enrolled. Exclusion criteria included the following: (1) history of allergy to pregabalin, (2) ASA physical status of III or higher, (3) history of chronic pain or regularly receiving analgesics, (4) pregnancy or breastfeeding, and (5) history of alcohol or drug abuse.
The patients were categorized into two groups through computer-generated randomization. The pregabalin group (group P, n=20) received 150mg of pregabalin orally 2 h before anesthetic induction. The control group (group C, n=20) received placebo tablets at the same time as group P. No other pre-anesthetic medications were administered. Upon arrival at the operating room, standard monitoring involving electrocardiography, pulse oximetry, non-invasive blood pressure monitoring, and bispectral index measurement, was performed. Anesthesia was induced intravenously using 1.5-2.5 mg/kg of propofol. Target-controlled infusion of 3-4 ng/mL of remifentanil and subsequently 0.6-1mg/kg of rocuronium were administered, followed by tracheal intubation. Anesthesia was maintained using sevoflurane and remifentanil in 50% oxygen with air, maintaining both hemodynamic stability (within 20% of the preoperative baseline values of mean blood pressure) and a bispectral index of 40-60. All patients received 30mg of ketorolac intravenously for postoperative pain and 0.3mg of ramosetron, a prophylactic antiemetic, during skin closure. Sevoflurane and remifentanil were stopped at the end of surgery, and the intraoperative total infusion dose of remifentanil was recorded. Residual neuromuscular paralysis was treated with intravenous administration of 0.2 mg/kg of pyridostigmine and 0.01mg/kg of glycopyrrolate.
In post-anesthesia care, fentanyl-based intravenous PCA (fentanyl at 30 μg/kg with a total volume of 60mL) was administered. The PCA device was programmed to deliver the drug at a basal infusion rate of 0.5mL/h, with a demand of 0.5mL with a lock-out period of 15min. All study medications and PCA devices were handled by an anesthesiologist blinded to group allocation. The postoperative pain intensity was evaluated using the visual analog scale (VAS; 0: no pain, 10: worst possible pain) at different postoperative time points (30min, 2 h, 4 h, and 24 h). Rescue analgesics (50 μg of fentanyl) were administered when the VAS score exceeded 4 or upon patient request. A rescue antiemetic (10mg of metoclopramide) was injected when any emetic episode developed or upon patient request. The total consumption of fentanyl in PCA, proportion of patients requiring rescue analgesics, and pregabalin-related side effects (e.g., dizziness, sedation, and dry mouth) were also noted during the 24 h postoperative period.
On the basis of preliminary studies, the effect size of consumption of fentanyl in PCA was 0.91. The sample size per group was determined to be 20 patients with α=0.05 and a power of 0.8 using G power. Considering the dropout rate, 44 patients were needed. Data are expressed as the mean±standard deviation or number (%). IBM SPSS version 23.0 (IBM Co., Armonk, NY, USA) was used for analysis and the Student t test, chi-squared test, and Fisher exact test were used. A p-value <0.05 was considered statistically significant.
A total of 44 patients were randomized, and the final analysis included data from 40 patients. Four patients were excluded because two underwent re-gastrectomy, one refused to participate, and one did not understand the VAS instrument. No statistical differences were observed in the demographic characteristics of patients or in the operative data, including intraoperative remifentanil consumption (Table 1). The postoperative pain intensity obtained from VAS scores did not exhibit significant differences at any postoperative time point (30min, 2 h, 4 h, and 24 h; Fig. 1). Consumption of fentanyl in PCA and the proportion of patients who required rescue analgesics at different time intervals (0-2 h, 2-4 h, and 4-24 h) did not differ significantly between the groups during the 24 h postoperative period (Table 2, 3). The groups did not differ in terms of the occurrence of dizziness, sedation, pruritus, and dry mouth (Table 4).
This study revealed that preoperative pregabalin (150mg) exerted no effect on intraoperative remifentanil consumption, post-surgical pain, or consumption of fentanyl in PCA within 24 h of gastrectomy. Pregabalin-related adverse effects, such as dizziness, sedation, and dry mouth, did not differ between the two groups.
A nociceptive stimulus caused by tissue injury during surgery releases multiple inflammatory mediators and neurotransmitters, which leads to peripheral sensitization, and subsequently produces central sensitization through afferent input to the central nervous system[8]. Although the mechanisms underlying progression from acute to chronic pain is not completely understood, controlling acute postoperative pain is critical because it poses a risk of chronic pain [9]. Acute postoperative pain is recognized to have early inflammatory and late neuropathic constituents [10]; therefore, preventing chronic pain involves not only alleviating inflammatory pain but also addressing neuropathic pain. On the basis of the pathophysiology of acute postoperative pain, using a single drug or intervention would not be sufficient as an effective treatment, particularly for moderate to severe pain.
Multimodal approaches to pain management have been demonstrated in many studies, with superior postoperative analgesia with fewer side effects than a single analgesic. Broadly, multimodal approaches for resolving pain involved pharmacological and non-pharmacological components; in addition, opioids, non-steroidal anti-inflammatory drugs, gabapentinoids, ketamine, dexmedetomidine, local anesthetics, regional anesthesia, and transcutaneous electrical nerve stimulation have been used in multimodal analgesic regimens [11-15]. Kehlet and Dahl demonstrated the effect of multimodal regimens in postoperative pain, and this approach, by combining medications with different mechanisms, achieved superior analgesia and fewer side effects [16].
Pregabalin has been extensively evaluated as part of multimodal regimens for pain. Pregabalin binds to calcium channels and subsequently modulates calcium influx and several excitatory neurotransmitters [17]. This mode of action can confer antiepileptic, anxiolytic, and analgesic effects. Moreover, the rapid absorption and linear pharmacokinetics of pregabalin after oral administration allow it to be used as a preemptive adjuvant as well as an acute postoperative analgesic during the perioperative period [18]. Many studies have examined the utility of pregabalin in post-surgical pain. Agarwal et al. reported that preoperative pregabalin reduced post-surgical pain and fentanyl requirement in patients undergoing laparoscopic cholecystectomy [19]. In a study by Rajappa et al., preoperative administration of 150mg of pregabalin was optimal for alleviating acute post-mastectomy morphine consumption [4]. However, some studies have failed to demonstrate analgesic benefits of pregabalin during the perioperative period. Jokera et al. reported no analgesic effect of preoperative use of pregabalin after laparoscopic hysterectomy [20]. Furthermore, Paech et al. did not observe stronger pain relief in patients administered pregabalin preoperatively [21]. In this study, we found no effect of 150mg of pregabalin on post-surgical pain or attenuation of fentanyl consumption in patients undergoing gastrectomy. These conflicting results concerning the utility of pregabalin could be due to differences in the dosage and regimen, severity of pain, and type of surgery.
We selected a single dose of pregabalin to identify any beneficial effect on post-gastrectomy pain. In pregabalin medication instructions, 150mg of pregabalin is suggested as the starting dose for treating neuropathic pain [17]. In the study of Esmat et al., both 150 and 300mg of pregabalin exerted similar analgesic effects on postoperative pain after laparoscopic cholecystectomy, but 300mg of pregabalin resulted in more side effects [22]. In the present study, we did not find any analgesic effects of 150mg of pregabalin on post-gastrectomy pain. These results might be due to the subtherapeutic doses of pregabalin used for the surgical population in our study. The larger doses on the occurrence of side effects should be cautiously examined.
This study had some limitations. First, we evaluated only a single dose of pregabalin. A study on different doses of pregabalin might be required to determine the benefits in postgastrectomy patients. Second, although power analysis was performed, the sample size was relatively small; additional studies with a larger sample size may reduce the risk of type II error.
In conclusion, preoperative administration of 150mg of pregabalin does not reduce postoperative pain or fentanyl consumption in the 24 h post-gastrectomy period.
REFERENCES
1. Apfelbaum JL, Chen C, Mehta SS, Gan TJ. Postoperative pain experience: results from a national survey suggest postoperative pain continues to be undermanaged. Anesth Analg. 2003; 97:534–40.

2. Rathmell JP, Wu CL, Sinatra RS, Ballantyne JC, Ginsberg B, Gordon DB, et al. Acute post-surgical pain management: a critical appraisal of current practice, December 2-4, 2005. Reg Anesth Pain Med. 2006; 31(4 Suppl 1):1–42.

3. Finnerup NB, Sindrup SH, Jensen TS. The evidence for pharmacological treatment of neuropathic pain. Pain. 2010; 150:573–81.

4. Rajappa GC, Vig S, Bevanaguddaiah Y, Anadaswamy TC. Efficacy of pregabalin as premedication for post-operative analgesia in vaginal hysterectomy. Anesth Pain Med. 2016; 6:e34591.

5. Hetta DF, Mohamed MA, Mohammad MF. Analgesic efficacy of pregabalin in acute postmastectomy pain: placebo controlled dose ranging study. J Clin Anesth. 2016; 34:303–9.

6. Cabrera Schulmeyer MC, de la Maza J, Ovalle C, Farias C, Vives I. Analgesic effects of a single preoperative dose of pregabalin after laparoscopic sleeve gastrectomy. Obes Surg. 2010; 20:1678–81.

7. Buvanendran A, Kroin JS, Della Valle CJ, Kari M, Moric M, Tuman KJ. Perioperative oral pregabalin reduces chronic pain after total knee arthroplasty: a prospective, randomized, controlled trial. Anesth Analg. 2010; 110:199–207.

9. Werner MU, Mjöbo HN, Nielsen PR, Rudin A. Prediction of postoperative pain: a systematic review of predictive experimental pain studies. Anesthesiology. 2010; 112:1494–502.
10. Kehlet H, Jensen TS, Woolf CJ. Persistent postsurgical pain: risk factors and prevention. Lancet. 2006; 367:1618–25.

11. Lavand’homme P, De Kock M, Waterloos H. Intraoperative epidural analgesia combined with ketamine provides effective preventive analgesia in patients undergoing major digestive surgery. Anesthesiology. 2005; 103:813–20.

12. Seib RK, Paul JE. Preoperative gabapentin for postoperative analgesia: a meta-analysis. Can J Anaesth. 2006; 53:461–9.

13. Capdevila X, Pirat P, Bringuier S, Gaertner E, Singelyn F, Bernard N, et al. Continuous peripheral nerve blocks in hospital wards after orthopedic surgery: a multicenter prospective analysis of the quality of postoperative analgesia and complications in 1,416 patients. Anesthesiology. 2005; 103:1035–45.
14. Tufanogullari B, White PF, Peixoto MP, Kianpour D, Lacour T, Griffin J, et al. Dexmedetomidine infusion during laparoscopic bariatric surgery: the effect on recovery outcome variables. Anesth Analg. 2008; 106:1741–8.

15. Bjordal JM, Johnson MI, Ljunggreen AE. Transcutaneous electrical nerve stimulation (TENS) can reduce postoperative analgesic consumption. A meta-analysis with assessment of optimal treatment parameters for postoperative pain. Eur J Pain. 2003; 7:181–8.

16. Kehlet H, Dahl JB. The value of “multimodal” or “balanced analgesia” in postoperative pain treatment. Anesth Analg. 1993; 77:1048–56.

17. Moore RA, Straube S, Wiffen PJ, Derry S, McQuay HJ. Pregabalin for acute and chronic pain in adults. Cochrane Database Syst Rev. 2009; 3:CD007076.

18. Shneker BF, McAuley JW. Pregabalin: a new neuromodulator with broad therapeutic indications. Ann Pharmacother. 2005; 39:2029–37.

19. Agarwal A, Gautam S, Gupta D, Agarwal S, Singh PK, Singh U. Evaluation of a single preoperative dose of pregabalin for attenuation of postoperative pain after laparoscopic cholecystectomy. Br J Anaesth. 2008; 101:700–4.

20. Jokela R, Ahonen J, Tallgren M, Haanpää M, Korttila K. A randomized controlled trial of perioperative administration of pregabalin for pain after laparoscopic hysterectomy. Pain. 2008; 134:106–12.

Fig. 1.
VAS score changes in both groups at postoperative 30 min, 2 h, 4 h, and 24 h . VAS, visual analog scale; group C, control group; group P, pregabalin group.
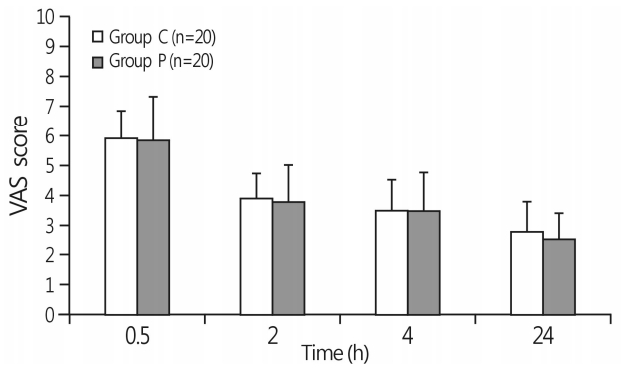
Table 1.
Patient characteristics
Table 2.
PCA fentanyl consumption and patients who required rescue analgesics
|
Patients group |
||
|---|---|---|
| Group C | Group P | |
| 0-2 h | 70.5±24.6 | 75.7±21.9 |
| 2-4 h | 58.5±21.6 | 68.2±25.3 |
| 4-24 h | 580.5±80.6 | 619.5±79.6 |
| 0-24 h | 709.5±107.3 | 763.5±82.5 |




 PDF
PDF ePub
ePub Citation
Citation Print
Print



 XML Download
XML Download