INTRODUCTION
The prevalence of atrial fibrillation (AF) is increasing as the population ages, and most elderly AF patients require anticoagulation treatment for stroke prevention as the associated risk increase greatly with age.
1)2) Nevertheless, oral anticoagulation remains underutilized due to the risk of bleeding.
3)4) Recently, the Edoxaban Low-Dose for Elder Care Atrial Fibrillation Patients (ELDERCARE-AF) trial reported that off-label underdosing of edoxaban (15 mg once daily) resulted in stroke risk reduction compared to placebo without increasing the risk of bleeding in very elderly patients.
5) Optimal anticoagulation for stroke prevention in very elderly AF patients who have additional risk factors for bleeding is challenging. Therefore, the ELDERCARE-AF trial concluded that, in very elderly patients who were not appropriate candidates for standard doses of oral anticoagulants, using a reduced dose is preferable to not using anticoagulation. However, these patients are also at high risk of stroke. Therefore, it is critical to achieve an appropriate balance between efficacy and safety. In the Effective Anticoagulation with Factor Xa Next Generation in Atrial Fibrillation-Thrombolysis In Myocardial Infarction 48 (ENGAGE AF-TIMI) trial, edoxaban was non-inferior to warfarin for stroke prevention in higher-dose regimens, while the event rate of ischemic stroke was greater for a lower-dose edoxaban regimen (LDER) than for warfarin.
6) In a pre-specified analysis of the ENGAGE AF-TIMI trial, the incidence of net clinical outcome of stroke/major bleeding/death in AF patients treated with a LDER was lower than for a higher-dose edoxaban regimen (HDER).
7)
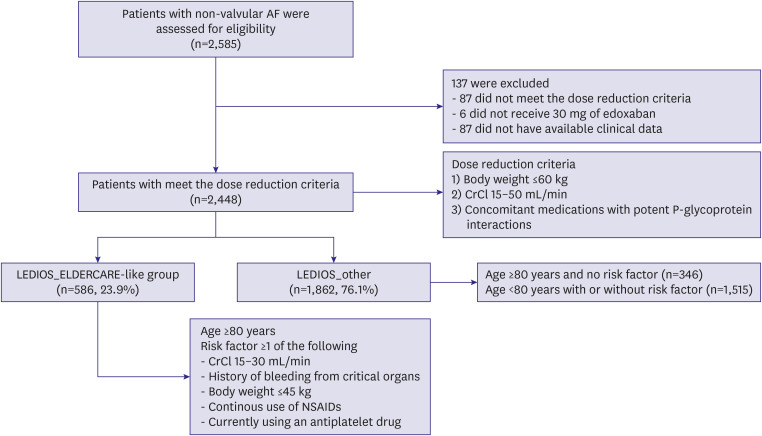
The aim of this study was to assess outcomes of on-label reduced dose edoxaban (30 mg) treatment of very elderly AF patients who had additional risk factors for bleeding in real-world practice.
DISCUSSION
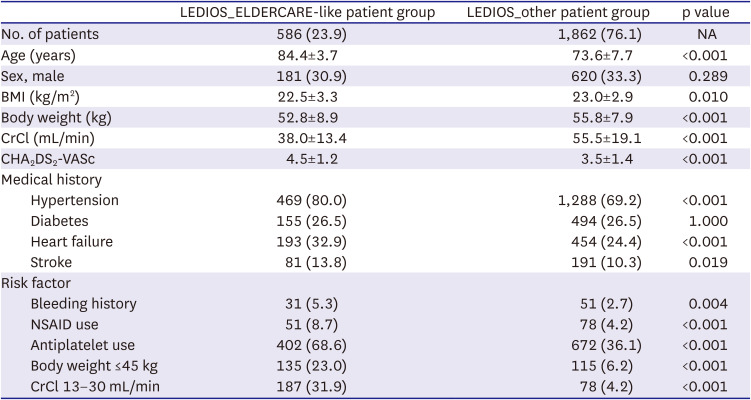
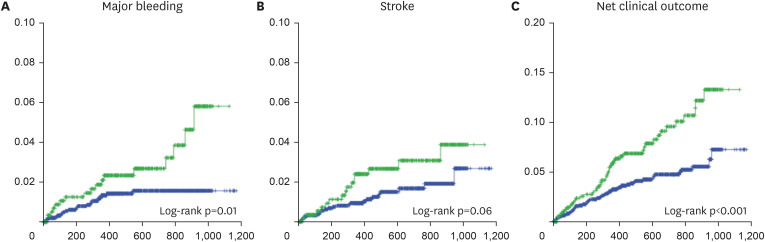
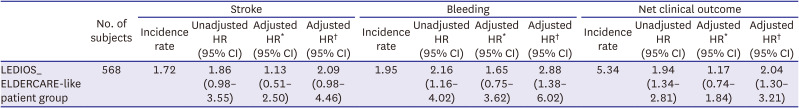
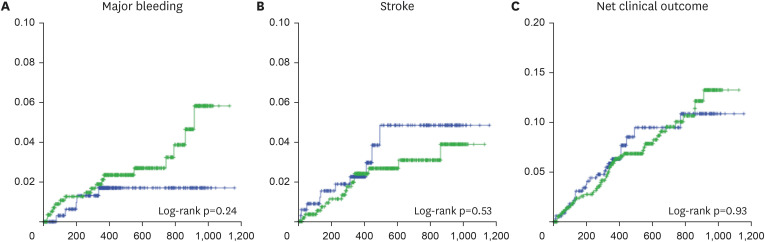
This study revealed the true high-risk group for bleeding events among very elderly AF patients, and the finding may guide safer use of oral anticoagulation in such patients while maintaining efficacy. The study analyses provided three main findings. First, bleeding risk was increased in patients in the LEDIOS_ELDERCARE-like group compared to other AF patients, while the ischemic stroke risk showed tendency to increase. Second, the risk was offset after adjustment for age. Third, in patients ≥80 years of age, bleeding risk and stroke event were similar according to the presence of bleeding risk factors.
Current guidelines recommend that older AF patients should receive oral anticoagulants to achieve more favorable outcomes.
10) However, oral anticoagulants are underutilized or underdosed in an off-label manner in patients at high risk of stroke.
1)11) Factors associated with label adherence were old age, history of bleeding, renal dysfunction, and low body weight. In the ELDERCARE trial, comorbidities associated with risk factors for bleeding were history of bleeding from a critical organ, CrCl 15–30 mL/min, body weight ≤45 kg, continuous use of NSAIDs, and currently using an antiplatelet drug. The risk of bleeding is often cited as the reason for off-label underdosing of NOACs or not prescribing oral anticoagulants. In our study, concomitant use of antiplatelet agents was observed in 68% of cases. The ENTRUST-AF PCI (edoxaban-based versus vitamin K antagonist-based antithrombotic regimen after successful coronary stenting in patients with AF) study reported that 60 mg edoxaban once daily plus an anti-platelet P2Y12 inhibitor was non-inferior for bleeding compared with a vitamin K antagonist combined with a P2Y12 inhibitor and aspirin.
12) However, the event rate for major bleeding in this study was 20.7%/yr in the edoxaban group. The event rate of major bleeding in the ELDERCARE trial was 3.3%/yr in the edoxaban group. These findings support the use of reduced-dose edoxaban in combination with antiplatelet agents in AF, especially for elderly patients. Indeed, in our study, the crude risk of bleeding and net clinical outcome rate were increased in very elderly AF patients who also had other risk factors for bleeding compared to other patients who did not have these risk factors, although the risk of stroke was similar, and neither bleeding nor stroke risk was significantly different in the 2 groups after adjustment for age. Moreover, when analyzing patients older than 80 years, there were no significant differences in outcomes according to the presence or absence of the risk factors examined. Therefore, in our study, the most powerful risk factor of increasing bleeding risk was age. Several reports have assessed the efficacy and safety of oral anticoagulants in patients older than 90 years.
13)14)15) In most studies, the use of NOACs was associated with a lower risk of clinical outcomes compared to that of warfarin. Furthermore, in very elderly patients over 90 years of age, NOACS were associated with a lower risk of composite adverse events compared to non-oral anticoagulant users, even for those with a history of intracranial hemorrhage (ICH), gastro-intestinal bleeding, or renal dysfunction. Brønnum Nielsen et al.
16) reported that the risk of ischemic stroke events after an ICH in patients with AF was greater than in non-ICH patients. Another study reported that warfarin use may be more beneficial than not using antithrombotic therapy with respect to net clinical outcome in patients who have AF and experience of ICH with a high stroke risk.
17) In other words, among AF patients, the high-risk group for bleeding is also at high risk of ischemic stroke, and careful consideration of the appropriate anticoagulation therapy is important in these patients.
Appropriate dosage of anticoagulants is important in patients at high risk of bleeding. Underdosing of apixaban in patients with normal or mildly impaired renal function was reported to be associated with a higher risk of stroke (HR of 4.87), but the risk of major bleeding events did not differ.
18) The ENGAGE AF-TIMI 48 trial, comparing two dose regimens of edoxaban with warfarin, showed that the rate of ischemic stroke was greater for the LDER (30/15 mg) compared with warfarin. In anticoagulation use, both efficacy and safety are important. Therefore, a net clinical outcome comprising stroke, bleeding, and mortality rate represents the balance of efficacy and safety. Steffel et al.
7) reported that a LDER reduced the primary net clinical outcome compared with a HDER, and a LDER may be considered in patients at high risk of bleeding. Based on this extension, the ELDERCARE-AF trial results suggest a lower dose of edoxaban in very elderly patients who had additional risk factors for bleeding. Our study results also showed increased risk of bleeding in our ELDERCARE-like patient group. However, the statistical significance of this result was reduced after adjustment for other variables including age. Age is known to be the most important risk factor for stroke. In addition, many studies have reported the safety of NOACs in elderly patients.
13)14) In our study, there were also no significant differences in outcomes among patients over 80 years of age according to the risk factors assessed. Based on these results, we suggest that an on-label dose of NOAC might be considered in elderly patients at high risk of bleeding, and close monitoring of bleeding events is important. In patients who have difficulty in maintaining a standard 30 mg dose of edoxaban, a reduced dose of 15 mg edoxaban might be an alternative treatment strategy.
This study had some limitations. First, this study was a non-interventional single-arm observational study. Therefore, we could not compare the outcomes with a no-antithrombotic group or a 15 mg edoxaban group. However, we can conclude that our ELDERCARE-like group was a truly at high risk of bleeding events. Furthermore, age is the most powerful risk factor for bleeding. Second, the incidence of each outcome assessed was relatively low. Given this was a real-world study, our study outcomes showed reasonable event rates. Further, a strength of our study was that we included patients indicated for on-label 30 mg of edoxaban in a real-world observational study.
In AF patients who had comorbidities associated with elevated risk of bleeding, major bleeding was significantly increased in very elderly patients. In addition, risk of stroke showed tendency to increase in same population. Bleeding and stroke risk was similar according to the presence of risk factors for bleeding among AF patients older than 80 years. Our findings suggest that not only the risk of bleeding, but also the risk of stroke is increased in very elderly patients. Effective anticoagulation therapy might be important in these high-risk population, and close observation of bleeding events might also be required.




 PDF
PDF Citation
Citation Print
Print



 XML Download
XML Download