Abstract
Purpose
This study aimed to assess the antimicrobial efficacy of an 810-nm infrared diode laser with indocyanine green (ICG) against Staphylococcus aureus on sandblasted, large grit, and acid-etched (SLA) titanium surfaces, comparing its effectiveness with alternative chemical decontamination modalities.
Materials and Methods
Biofilms of S. aureus ATCC 25923 were cultured on SLA titanium disks for 48 hours. The biofilms were divided into five treatment groups: control, chlorhexidine gluconate (CHX), tetracycline (TC), ICG, and 810-nm infrared diode laser with ICG (ICG-PDT). After treatment, colony-forming units were quantified to assess surviving bacteria, and viability was confirmed through confocal laser-scanning microscope (CLSM) imaging.
Results
All treated groups exhibited a statistically significant reduction in S. aureus (P<0.05), with notable efficacy in the CHX, TC, and ICG-PDT groups (P<0.01). While no statistical difference was observed between TC and CHX, the ICG-PDT group demonstrated superior bacterial reduction. CLSM images revealed a higher proportion of dead bacteria stained in red within the ICG-PDT groups.
초록
목적
이 연구의 목적은 거친 티타늄 표면에 형성된 Staphylococcus aureus 바이오필름에 대해 인도시아닌 그린을 활용한 광역학치료(photodynamic therapy; PDT)의 항미생물 효과를 평가하고, 이를 임상에서 널리 사용되는 다른 화학 처치 방법과 비교하는 것이다.
연구 재료 및 방법
멸균된 거친 표면 티타늄 디스크에 S. aureus ATCC 25923을 접종한 후 48시간 동안 배양하여 바이오필름을 형성하였다. 실험은 대조군, 클로르헥시딘군(CHX), 테트라싸이클린군(TC), 인도시아닌 그린군(ICG), 인도시아닌 그린을 활용한 광역학치료군(ICG-PDT)으로 구분하여 진행하였다. 군에 따른 처리 후 세균을 배양하여 세균 수(colony forming unit; CFU)를 계산하고, 바이오필름을 공초점 현미경을 통해 분석하였다. 통계 분석은 CFU값을 로그 값으로 변환한 후 분산 분석을 시행하였다.
A recent review revealed that the weighted mean prevalences for peri-implantitis were 9.25% and 19.83% based on implant and subject respectively. Similarly, the weighted mean prevalences for peri-implant mucositis were 29.48% and 46.83% based on implant and subject respectively. The study found that peri-implant diseases were prevalent and the prevalence of peri-implantitis increased over time.1 Traditional treatment approaches involve mechanical debridement using instruments such as curettes, ultrasonic scalers, and air powder abrasion. These methods are often combined with chemical treatments using substances like citric acid, hydrogen peroxide, ethylene-diamine-tetraacetic acid, chlorhexidine gluconate, and local or systemic antibiotics.2-4
However, these treatments may be associated with undesirable outcomes, including implant surface damage, chemotoxicity to surrounding tissues, and the emergence of antibiotic-resistant bacterial strains.3-5 To address these challenges, antimicrobial photodynamic therapy (PDT) has been proposed as an alternative for treating peri-implant diseases, aiming to mitigate the side effects associated with conventional treatment modalities.5 PDT is a non-invasive photochemical treatment modality designed to eliminate periodontal pathogens using low-level laser light (wavelength range: 650 - 940 nm) with a photosensitizer. Photosensitizers absorb specific wavelengths, converting light into energy that generates reactive oxygen species (ROS). ROS exert a cytotoxic impact by damaging DNA, inhibiting cell-wall synthesis, and modifying cell membrane proteins or potassium ions.5 PDT offers several advantages, including the absence of bacterial resistance development, targeted antibacterial efficacy at the treatment site, and minimal damage to adjacent healthy tissues due to its localized effect.6,7
Recommended photosensitizers include toluidine blue, methylene blue, and indocyanine green (ICG), with ICG noted for its optimal peak absorption at 805 - 810 nm.8,9 Some studies have suggested that the mechanism of diode laser with ICG (ICG-PDT) involves not only the photodynamic effect that produces ROS but also the photothermal effect, inducing cell destruction by elevating intercellular temperature.9-11 Bashir et al.11 demonstrated the effectiveness of ICG-PDT in destroying periodontopathogenic cells and improving clinical periodontal parameters in chronic periodontitis.
The sequence of biofilm formation and microbial species involved in implant surface colonization resembles those observed on teeth surfaces.12,13 Surface characteristics, including roughness and surface free energy of implant surfaces, significantly influence biofilm formation.12 Previous studies reported the highest bacterial adhesion on rough implant surfaces, particularly those represented by the sandblasted, large grit, and acid-etched (SLA) surface.14-16 Staphylococcus aureus has been implicated in infections associated with implanted medical devices and exhibits a pronounced affinity for titanium surfaces.17-19 As an early colonizer, it facilitates the subsequent adhesion of other bacteria, creating a conducive environment.20 It was demonstrated that high levels of S. aureus were identified in peri-implantitis lesions and associated with the presence of suppuration and bleeding on probing.21 Consequently, disrupting S. aureus biofilm is imperative for preventing peri-implant disease.
Although several in vitro studies demonstrated the efficacy of ICG-PDT in reducing S. aureus,22,23 the effectiveness of ICG-PDT against S. aureus biofilm specifically on the SLA rough titanium surface has not been evaluated. Therefore, the purpose of this in vitro study is to assess the antimicrobial efficacy of an infrared diode laser utilizing ICG against S. aureus on SLA titanium surfaces, comparing its effectiveness with alternative chemical decontamination modalities.
Titanium disks (Φ 8.0 mm, height 2.5 mm, Shinhung, Seoul, Korea) underwent sandblasting and acid etching (SLA). These disks were then covered with a putty-type vinyl polysiloxane (GC Corporation, Tokyo, Japan), leaving one surface exposed for biofilm formation. Subsequently, the disks underwent sonication, soaking in 70% ethanol, and autoclaving at 121°C for 15 m.
S. aureus ATCC 25923 was cultured on a blood agar plate for 24 h and inoculated into 5 mL tryptic soy broth (TSB) at 37°C to create a suspension. The suspension was adjusted to 1 × 108 colony forming unit per mL (CFU/mL) using a spectrophotometer (Smart Plus 2700; Young-woo instrument, Seoul, Korea). Ti disks were placed in a 12-well plate and inoculated with 100 μL bacterial suspensions and 2 mL TSB to allow biofilm formation for 48 h at 37°C. The incubation time was determined based on the growth curve of S. aureus on the SLA titanium surface revealed in the results of a previous study.24 Based on the biofilm growth curve, the titanium disks placed in the 12-well plate with 2 mL TSB and 100 μl bacterial suspension were incubated for 48 h.
After 48 h of incubation, disks were gently washed twice with 2 mL phosphate-buffered saline (PBS) and divided into five groups (Table 1). Each group consisted of six disks, and the experiment was repeated twice. The control group underwent a 30 s wash with 100 μL PBS. The chlorhexidine gluconate (CHX) group received a 30 s treatment with 0.1% CHX (Bukwang Pharm. Co., Seoul, Korea), the Tetracycline (TC) group was treated with 50 mg/mL TC (Teracyclin®, Chong Kun Dang, Seoul, Korea) solution for 30 s, the indocyanine green (ICG) group received a 5 m treatment with 1 mg/mL ICG, and the photodynamic therapy (ICG-PDT) group underwent a 5 m treatment with 1 mg/mL ICG activated with infrared diode laser irradiation (Laserland, Hubei, China) at 810-nm wavelength, 300 mW power, 24 J/cm2 energy density, irradiation time of 30 s. Irradiation was performed in the dark under aseptic conditions, and disks were shielded to prevent light transmission. After treatment, disks were washed twice with PBS, and biofilm was dispersed by sonication for 15 s. A 1 : 100 dilution was made using PBS, and two blood agar plates were inoculated. Colony-forming units (CFU) were counted using an automated colony counter (IUL, Barcelona, Spain).
To assess bacterial cell viability after treatment, a LIVE/DEAD™ BacLight™ Bacterial Viability Kit (Molecular probes Inc. Eugene, USA) was utilized following the manufacturer’s instructions. Samples were visualized using a confocal laser-scanning microscope (CLSM; TCS SP8 STED, Leica Microsystems, Mannheim, Germany). Treatment effects were evaluated by analyzing merged images with an image processing program (LAS X, Leica Microsystems) at a 10× magnification.
Antimicrobial efficacy was determined by dividing the mean CFU value of the experimental group by that of the control group, with bacterial colony count expressed as a log-transformed value. Groups were compared using one-way analysis of variance, followed by Tukey’s HSD test. Statistical analysis was performed using SPSS software (version 28, IBM, Armonk, USA), with the level of significance set at P < 0.05
Table 2 presents the bacterial reduction rates for each group. All treatment groups exhibited a significant reduction in bacterial viability. Notably, the CHX, TC, and ICG-PDT groups demonstrated a bacterial reduction rate exceeding 90%. When comparing groups based on log-transformed values (Fig. 1), there was a statistically significant decrease in bacterial viability in the chemical disinfection groups compared to the control group (P < 0.05), with a particularly pronounced effect in the CHX, TC, and ICG-PDT groups (P < 0.01) (Table 2). In the comparison among these three groups, the ICG-PDT group displayed a significantly greater reduction in CFU compared to the TC group (P = 0.035). However, there was no significant difference between the ICG-PDT and CHX groups, although there was a trend toward increased CFU reduction in the mean value (P = 0.520).
In CLSM images, live bacteria were stained in green, while dead bacteria were stained in red (Fig. 2). The control group’s image appeared green, indicating a high proportion of live bacteria. In comparison with the control group, all treatment groups exhibited a trend toward a lower proportion of live bacteria and a higher proportion of dead bacteria. Among these groups, the ICG-PDT group’s images revealed a relatively higher number of dead bacteria stained in red compared to the other groups.
This in vitro study aimed to assess the antimicrobial effectiveness of ICG-PDT against S. aureus biofilm on SLA titanium surfaces, comparing it with CHX and TC. The results indicate that ICG-PDT is a highly effective method for reducing S. aureus biofilm on SLA titanium surfaces.
Effective management of peri-implant biofilm is crucial in the treatment of peri-implant disease. Previous studies have shown that ICG-PDT significantly reduces pathogenic bacteria, including Porphyromonas gingivalis, Streptococcus mutans, Enterococcus faecalis, and Aggregatibacter actinomycetemcomitans in vitro.25-27 Moreover, systematic reviews have reported improved clinical periodontal parameters with the adjunctive use of ICG-PDT in non-surgical periodontal therapy for chronic periodontitis.11
In this study, the antimicrobial efficacy of ICG-PDT against S. aureus biofilm on SLA titanium surface was evaluated through bacterial viability tests and CLSM. The results demonstrated a significant antimicrobial effect of ICG-PDT compared to the control, TC, and ICG groups. CLSM images further revealed a higher proportion of dead bacteria stained in red in the ICG-PDT group, indicating its efficacy in disrupting S. aureus biofilm on the SLA titanium surface. While the statistical comparison between the ICG-PDT and CHX groups did not reach significance, there was a trend towards a greater reduction in mean value in the ICG-PDT group. This observation aligns with previous studies demonstrating significant reduction with CHX or comparable reduction between CHX and ICG-PDT groups, albeit with variations in experimental methods.28,29
In this study, there was a higher proportion of dead bacteria in the ICG-PDT group in CLSM images. This result might be explained by photothermal effect which penetrate even in deeper layer of biofilm in contrast of CHX, penetrating only limited depth of the biofilm.29 ICG transformed most of the absorbed energy of laser into heat, increasing the penetration depth of effect.30 In previous study, similar result was reported that the antimicrobial effect of ICG-PDT highly penetrated within the mature biofilm despite the comparable CFU values between CHX and ICG-PDT.29
The study also considered the impact of implant macro-design and surface roughness on treatment efficacy. While these factors enhance osseointegration, the rough surface may act as a bacterial reservoir.31,32 An in vitro study reported that implant macrostructure significantly influenced the access of the mechanical decontamination devices and in turn its efficacy.33 Therefore, adjunctive antimicrobial approaches such as antiseptic, or local or systematic antibiotics should be considered to improve the treatment outcomes and to prevent bacterial colonization. Both CHX and TC groups in this study demonstrated a statistically significant reduction in bacterial viability compared to the control group, highlighting the efficacy of chemical decontamination. However, there were no significant differences between CHX and TC groups, suggesting a lack of superiority for one treatment modality over the other.4
The potential complications of implant surface decontamination include mechanical damage, chemotoxicity, and the emergence of resistant bacteria with antibiotics.4 ICG-PDT offers advantages such as low chemotoxicity, ease of handling, rapid elimination, and minimal side effects.10 The generated ROS in ICG-PDT have a limited migration depth, minimizing damage to sound tissue.5 These facts advocate the use of ICG-PDT in treatment of peri-implant disease with less side effect possibility.
Despite its strengths, the study has limitations. The exclusion of other treatment options and the absence of mechanical intervention limit the generalizability of the findings. Moreover, the single bacterial species (S. aureus) and titanium disk used may not fully represent the complexity of clinical situations. Further in vivo or clinical studies are warranted to validate the effectiveness of various decontamination methods against multi-species biofilm on implant surfaces.
Despite the limitations, the superior effect of ICG-PDT suggests its potential clinical relevance in peri-implant disease treatment. In practice, ICG-PDT could effectively disrupt biofilm with minimized side effects, limiting irradiation to specific areas. Understanding the mechanism of PDT and the etiology of peri-implant disease is essential for effective prevention and treatment.
Within the limitations, ICG-PDT demonstrated effective disruption of S. aureus biofilm on SLA titanium surfaces. These results support the consideration of ICG-PDT for implant surface decontamination. Further research exploring alternative decontamination methods and assessing the clinical impact of ICG-PDT on peri-implant disease lesions is warranted.
References
1. Lee CT, Huang YW, Zhu L, Weltman R. 2017; Prevalences of peri-implantitis and peri-implant mucositis: systematic review and meta-analysis. J Dent. 62:1–12. DOI: 10.1016/j.jdent.2017.04.011. PMID: 28478213.

2. Lindhe J, Meyle J. 2008; Peri-implant diseases: Consensus Report of the Sixth European Workshop on Periodontology. J Clin Periodontol. 35(8 Suppl):282–5. DOI: 10.1111/j.1600-051X.2008.01283.x. PMID: 18724855.

3. Giffi R, Pietropaoli D, Mancini L, Tarallo F, Sahrmann P, Marchetti E. 2023; The efficacy of different implant surface decontamination methods using spectrophotometric analysis: an in vitro study. J Periodontal Implant Sci. 53:295–305. DOI: 10.5051/jpis.2203500175. PMID: 36731864. PMCID: PMC10465813.

4. Claffey N, Clarke E, Polyzois I, Renvert S. 2008; Surgical treatment of peri-implantitis. J Clin Periodontol. 35(8 Suppl):316–32. DOI: 10.1111/j.1600-051X.2008.01277.x. PMID: 18724859.

5. Sculean A, Deppe H, Miron R, Schwarz F, Romanos G, Cosgarea R. 2021; Effectiveness of Photodynamic Therapy in the Treatment of Periodontal and Peri-Implant Diseases. Monogr Oral Sci. 29:133–43. DOI: 10.1159/000510189. PMID: 33427227.

6. Konopka K, Goslinski T. 2007; Photodynamic therapy in dentistry. J Dent Res. 86:694–707. DOI: 10.1177/154405910708600803. PMID: 17652195.

7. Cho K, Lee SY, Chang BS, Um HS, Lee JK. 2015; The effect of photodynamic therapy on Aggregatibacter actinomycetemcomitans attached to surface-modified titanium. J Periodontal Implant Sci. 45:38–45. DOI: 10.5051/jpis.2015.45.2.38. PMID: 25932337. PMCID: PMC4415000.

8. Shirata C, Kaneko J, Inagaki Y, Kokudo T, Sato M, Kiritani S, Akamatsu N, Arita J, Sakamoto Y, Hasegawa K, Kokudo N. 2017; Near-infrared photothermal/photodynamic therapy with indocyanine green induces apoptosis of hepatocellular carcinoma cells through oxidative stress. Sci Rep. 7:13958. DOI: 10.1038/s41598-017-14401-0. PMID: 29066756. PMCID: PMC5654824.

9. Boehm TK, Ciancio SG. 2011; Diode laser activated indocyanine green selectively kills bacteria. J Int Acad Periodontol. 13:58–63.
10. Monzavi A, Chinipardaz Z, Mousavi M, Fekrazad R, Moslemi N, Azaripour A, Bagherpasand O, Chiniforush N. 2016; Antimicrobial photodynamic therapy using diode laser activated indocyanine green as an adjunct in the treatment of chronic periodontitis: A randomized clinical trial. Photodiagnosis Photodyn Ther. 14:93–7. DOI: 10.1016/j.pdpdt.2016.02.007. PMID: 26921460.

11. Bashir NZ, Singh HA, Virdee SS. 2021; Indocyanine green-mediated antimicrobial photodynamic therapy as an adjunct to periodontal therapy: a systematic review and meta-analysis. Clin Oral Investig. 25:5699–710. DOI: 10.1007/s00784-021-03871-2. PMID: 33710461. PMCID: PMC8443506.

12. Subramani K, Jung RE, Molenberg A, Hammerle CH. 2009; Biofilm on dental implants: a review of the literature. Int J Oral Maxillofac Implants. 24:616–26.
13. Mombelli A, Décaillet F. 2011; The characteristics of biofilms in peri-implant disease. J Clin Periodontol. 38 Suppl 11:203–13. DOI: 10.1111/j.1600-051X.2010.01666.x. PMID: 21323716.

14. Wu-Yuan CD, Eganhouse KJ, Keller JC, Walters KS. 1995; Oral bacterial attachment to titanium surfaces: a scanning electron microscopy study. J Oral Implantol. 21:207–13.
15. Roehling S, Astasov-Frauenhoffer M, Hauser-Gerspach I, Braissant O, Woelfler H, Waltimo T, Kniha H, Gahlert M. 2017; In Vitro Biofilm Formation on Titanium and Zirconia Implant Surfaces. J Periodontol. 88:298–307. DOI: 10.1902/jop.2016.160245. PMID: 27712464.

16. Almaguer-Flores A, Olivares-Navarrete R, Wieland M, Ximénez-Fyvie LA, Schwartz Z, Boyan BD. 2012; Influence of topography and hydrophilicity on initial oral biofilm formation on microstructured titanium surfaces in vitro. Clin Oral Implants Res. 23:301–7. DOI: 10.1111/j.1600-0501.2011.02184.x. PMID: 21492236. PMCID: PMC4287405.
17. Christensen GD, Baddour LM, Hasty DL, Lowrance JH, Simpson WA. Bisno AL, Waldvogel FA, editors. Microbial and foreign body factors in the pathogenesis of medical device infections. Infections associated with indwelling medical devices. Washington: American Society for Microbiology;1989. p. 27–59.
18. Lister JL, Horswill AR. 2014; Staphylococcus aureus biofilms: recent developments in biofilm dispersal. Front Cell Infect Microbiol. 4:178. DOI: 10.3389/fcimb.2014.00178. PMID: 25566513. PMCID: PMC4275032.

19. Kronström M, Svensson B, Erickson E, Houston L, Braham P, Persson GR. 2000; Humoral immunity host factors in subjects with failing or successful titanium dental implants. J Clin Periodontol. 27:875–82. DOI: 10.1034/j.1600-051x.2000.027012875.x. PMID: 11140553.

20. Persson GR, Renvert S. 2014; Cluster of bacteria associated with peri-implantitis. Clin Implant Dent Relat Res. 16:783–93. DOI: 10.1111/cid.12052. PMID: 23527870.

21. Salvi GE, Fürst MM, Lang NP, Persson GR. 2008; One-year bacterial colonization patterns of Staphylococcus aureus and other bacteria at implants and adjacent teeth. Clin Oral Implants Res. 19:242–8. DOI: 10.1111/j.1600-0501.2007.01470.x. PMID: 18177429.
22. Topaloglu N, Gulsoy M, Yuksel S. 2013; Antimicrobial photodynamic therapy of resistant bacterial strains by indocyanine green and 809-nm diode laser. Photomed Laser Surg. 31:155–62. DOI: 10.1089/pho.2012.3430. PMID: 23402392.

23. Wong TW, Liao SZ, Ko WC, Wu CJ, Wu SB, Chuang YC, Huang IH. 2019; Indocyanine Green - Mediated Photodynamic Therapy Reduces Methicillin-Resistant Staphylococcus aureus Drug Resistance. J Clin Med. 8:411. DOI: 10.3390/jcm8030411. PMID: 30934605. PMCID: PMC6463108.

24. Park GH, Lee SY, Lee JB, Chang BS, Lee JK, Um HS. 2023; Effect of photodynamic therapy according to differences in photosensitizers on Staphylococcus aureus biofilm on titanium. Photodiagnosis Photodyn Ther. 41:103317. DOI: 10.1016/j.pdpdt.2023.103317. PMID: 36738904.

25. Beytollahi L, Pourhajibagher M, Chiniforush N, Ghorbanzadeh R, Raoofian R, Pourakbari B, Bahador A. 2017; The efficacy of photodynamic and photothermal therapy on biofilm formation of Streptococcus mutans: An in vitro study. Photodiagnosis Photodyn Ther. 17:56–60. DOI: 10.1016/j.pdpdt.2016.10.006. PMID: 27769914.

26. Golmohamadpour A, Bahramian B, Khoobi M, Pourhajibagher M, Barikani HR, Bahador A. 2018; Antimicrobial photodynamic therapy assessment of three indocyanine green-loaded metal-organic frameworks against Enterococcus faecalis. Photodiagnosis Photodyn Ther. 23:331–8. DOI: 10.1016/j.pdpdt.2018.08.004. PMID: 30077652.

27. Fekrazad R, Karamifar K, Bahador A. 2016; Comparison of antibacterial effect of photodynamic therapy using indocyanine green (Emundo) with 2% metronidazole and 2% chlorhexidine gel on Porphyromonas gingivalis (an in-vitro study). Photodiagnosis Photodyn Ther. 15:28–33. DOI: 10.1016/j.pdpdt.2016.04.003. PMID: 27129870.

28. Shim SH, Lee SY, Lee JB, Chang BS, Lee JK, Um HS. 2022; Antimicrobial photothermal therapy using diode laser with indocyanine green on Streptococcus gordonii biofilm attached to zirconia surface. Photodiagnosis Photodyn Ther. 38:102767. DOI: 10.1016/j.pdpdt.2022.102767. PMID: 35182778.

29. Burchard T, Karygianni L, Hellwig E, Follo M, Wrbas T, Wittmer A, Vach K, Al-Ahmad A. 2019; Inactivation of oral biofilms using visible light and water-filtered infrared A radiation and indocyanine green. Future Med Chem. 11:1721–39. DOI: 10.4155/fmc-2018-0522. PMID: 31368351.

30. Hopp M, Biffar R. 2013; Photodynamic therapies - blue versus green. Laser. 1:10–25.
31. Albrektsson T, Wennerberg A. 2019; On osseointegration in relation to implant surfaces. Clin Implant Dent Relat Res. 21 Suppl 1:4–7. DOI: 10.1111/cid.12742. PMID: 30816639.

32. Lin HY, Liu Y, Wismeijer D, Crielaard W, Deng DM. 2013; Effects of oral implant surface roughness on bacterial biofilm formation and treatment efficacy. Int J Oral Maxillofac Implants. 28:1226–31. DOI: 10.11607/jomi.3099. PMID: 24066312.

33. Sanz-Martín I, Paeng K, Park H, Cha JK, Jung UW, Sanz M. 2021; Significance of implant design on the efficacy of different peri-implantitis decontamination protocols. Clin Oral Investig. 25:3589–97. DOI: 10.1007/s00784-020-03681-y. PMID: 33170374.

Fig. 1
Comparison of log-transformed value of CFU/mL.
* P < 0.05: when comparing control with ICG, and TC with ICG-PDT.
** P < 0.01: when comparing control and ICG with CHX, TC, and ICG-PDT.
CHX: chlorhexidine; TC: tetracycline; ICG: indocyanine green; ICG-PDT: photodynamic therapy using ICG.
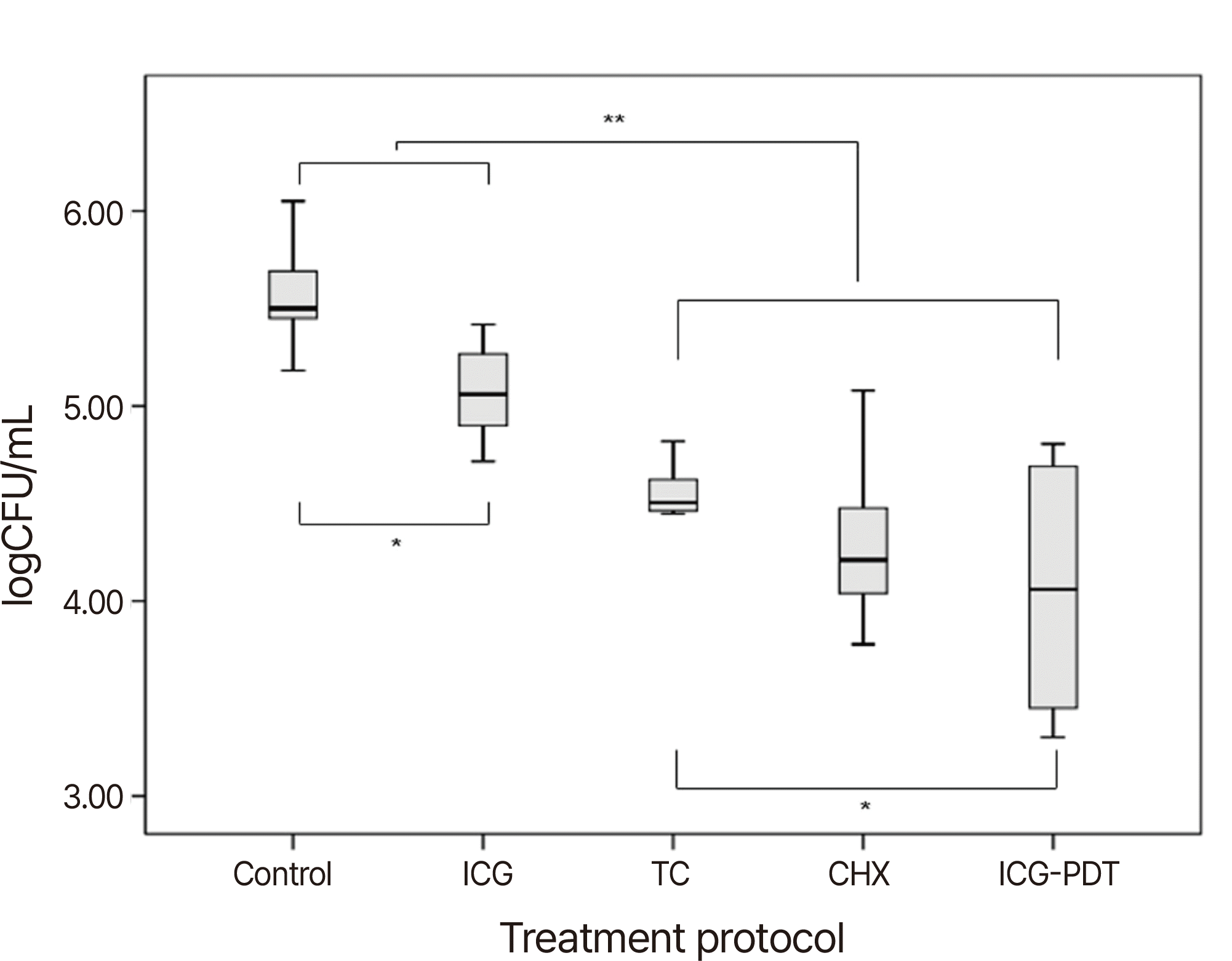
Fig. 2
Confocal laser-scanning microscope images of S. aureus biofilm on Ti disk (original magnification, 10x). Live and dead bacteria are stained green and red, respectively. Among these groups, the ICG-PDT group’s images revealed a relatively higher number of dead bacteria stained in red compared to the other groups.
(A) Control group, (B) Chlorhexidine group (CHX), (C) Tetracycline group (TC), (D) Indocyanine green group (ICG), (E) Photodynamic therapy using ICG group (ICG-PDT).




 PDF
PDF Citation
Citation Print
Print



 XML Download
XML Download