Abstract
With the increasing prevalence of heart failure and end-stage lung disease, there is a sustained interest in expanding the donor pool to alleviate the thoracic organ shortage crisis. Efforts to extend the standard donor criteria and to include donation after circulatory death have been made to increase the availability of suitable organs. Studies have demonstrated that outcomes with extended-criteria donors are comparable to those with standard-criteria donors. Another promising approach to augment the donor pool is the improvement of organ preservation techniques. Both ex vivo lung perfusion (EVLP) for the lungs and the Organ Care System (OCS, TransMedics) for the heart have shown encouraging results in preserving organs and extending ischemia time through the application of normothermic regional perfusion. EVLP has been effective in improving marginal or borderline lungs by preserving and reconditioning them. The use of OCS is associated with excellent short-term outcomes for cardiac allografts and has improved utilization rates of hearts from extended-criteria donors. While both EVLP and OCS have successfully transitioned from research to clinical practice, the costs associated with commercially available systems and consumables must be considered. The ex vivo perfusion platform, which includes both EVLP and OCS, holds the potential for diverse and innovative therapies, thereby transforming the landscape of thoracic organ transplantation.
Thoracic organ transplantation is the standard of care for end-stage heart and lung disease [1,2]. The world’s first heart transplant was performed by Christiaan Barnard in December 1967 at Groote Schuur Hospital in Cape Town [3], while James D. Hardy and Webb conducted their first lung transplant at the University of Mississippi Hospital on June 11, 1963 [4]. Korea’s first successful single lung transplantation took place in 1996. Although 157 lung transplants were performed in 2019, 277 patients remained on the waiting list, highlighting a significant disparity between the demand for and the availability of donor lungs [5,6]. Korean waitlist mortality for lung transplants increased by 7.9% from 2018, with the death of 82 listed candidates [5]. The first successful heart transplantation in Korea was carried out in 1992 [7]. Up until 2020, only 174 of the 774 patients (22.5%) on the waitlist in Korea have received donor hearts [8]. The global acceptance rate of thoracic organs from brain-dead patients is approximately 23%, and the annual waiting list mortality rate for patients with critical heart and lung failure has consistently been between 10% and 20% [9–11].
To address the shortage of organs, the International Society for Heart and Lung Transplantation (ISHLT) has broadened the ideal donor criteria to include extended-criteria donors (ECDs) and donation after circulatory death (DCD) [12,13]. Additionally, techniques such as ex vivo lung perfusion (EVLP) and the Organ Care System (OCS, TransMedics) are being employed to recondition and repair organs. Research has demonstrated that transplants using ECDs yield acceptable outcomes, though they may be less than optimal, or at times, noninferior [14]. These results may be attributed not only to improved donor management but also to advancements in clinical expertise and recipient care, which have collectively contributed to redefining the acceptance criteria for thoracic organ donation.
A list of extended donor criteria for heart and lungs is presented as the following [15]: extended heart criteria; (1) age >55 years, (2) cold ischemic time >360 minutes, (3) donor/recipient weight ratio <0.80 or ≥1.30, (4) hepatitis C positivity, (5) drug use history, (6) renal insufficiency (serum creatinine >2.0 mg/dL), (7) left ventricular ejection fraction <50%, and (8) DCD. Extended lung criteria: (1) age ≥55 years, (2) infiltrates on chest radiograph, (3) PaO2/FiO2 ratio (P/F ratio) <300 at a fraction of inspired oxygen (FiO2) of 1.0 and positive end-expiratory pressure (PEEP) of 5 cmH2O, (4) purulent secretions on bronchoscopy or evidence of aspiration/sepsis, and (5) cigarette use history (>20 pack-years).
Both EVLP and OCS are machine perfusion technologies that enable the preservation, resuscitation, assessment, repair, and reconditioning of cardiac and pulmonary allografts outside the body prior to scheduled transplantation [16]. This review compiles findings on the use of EVLP and OCS in thoracic organ transplantation. It aims to provide insights into the development, techniques, and outcomes of EVLP and OCS.
The first successful lung transplant, performed by Hardy and Webb in 1963, utilized a DCD donor, with the pulmonary graft sourced from an individual who had succumbed to myocardial infarction [4]. The introduction of Maastricht's classification of DCD in 1995, along with the significant contributions of Thomas M. Egan, spurred increased interest in the use of DCD donors [17–19].
Love et al. [20] reported the first successful case of a single lung transplantation from a controlled DCD in 1995. Outcomes of lung transplants from DCD donors have been found to be comparable to those from donation after brain death (DBD) donors [21–23]. As the use of DCD donors gains momentum, there is a growing interest in the use of EVLP for lung evaluation and reconditioning.
The concept of ex vivo organ perfusion was conceived as early as 1935 by Carrel and Lindbergh [24]. In 1970, Jirsch et al. [25] attempted to perfuse canine lungs for storage prior to transplantation at the University of Alberta, Canada, but their efforts were unsuccessful. Subsequent attempts at ex vivo perfusion were similarly disappointing.
Steen and colleagues in Lund developed the world's first EVLP circuit for clinical use, which facilitated the improved assessment of lungs from donors, particularly those from uncontrolled DCD. In 2001, Stig Steen successfully performed a single lung transplant from an uncontrolled DCD following EVLP. Along with his team, he reported the first successful lung transplant from a marginal donor after perfusion and reconditioning using EVLP in 2005.
The commercially available EVLP systems—OCS Lung, XPS (XVIVO Perfusion AB), Lung Assist (Organ Assist), and Vivoline LS1 (Vivoline Medical AB)—as well as other indigenous EVLP systems, are based on three strategies: Lund, Toronto, and Hannover. Details of the various EVLP strategies are provided in Table 1. The general technique and procedure for conducting EVLP, subsequent to the standard harvesting of donor lungs, are described as follows. The indications of EVLP for both brain-dead donors and DCD donors are: (1) best P/F ratio <300 mmHg; (2) signs of pulmonary edema, either on chest X-ray or physical examination at the donor site; (3) poor lung compliance in the examination during the procurement operation; (4) high-risk history, such as >10 units of blood transfusion or questionable history of aspiration; (5) DCD donors with a >60-minute interval from withdrawal life support to cardiac arrest; and (6) pulmonary embolism or focal consolidations due to infection, aspiration, or lung contusion [26,27]. Donor lungs with significant consolidation due to infection, trauma, or gross aspiration deemed unsuitable for transplantation are excluded. The principles of EVLP are as follows: (1) gradual rewarming to achieve normothermia and gradual cooling later; (2) gradual increase in the flow rate in EVLP as the lungs are rewarmed based on the donor’s predicted cardiac output; (3) protective lung ventilation; and (4) perfusate with increased colloid osmotic pressure [28].
All EVLP circuits in the world are based on the Lund group’s design. The core components of the circuit include a pump that circulates perfusate through a leukocyte filter and an oxygenator before reaching the lungs via a pulmonary artery cannula. The pulmonary venous return flows into the reservoir either through the left atrium (LA) or a closed LA cannula, allowing the perfusate to be recirculated (Fig. 1). An endotracheal tube is inserted for ventilation at a temperature of 32 °C [26]. The circuit is primed with 2.0 L of Steen solution (Toronto and Lund) or OCS/Perfadex (XVIVO) solution (Hannover) with 500 mg of methylprednisolone 3,000 IU of unfractionated heparin, and an antibiotic as additives. Isotonic trometamol is added to maintain the pH between 7.35 and 7.45 in the Lund protocol [29].
The LA cuff is trimmed and sewn to the LA cannula using 4-0 polypropylene running sutures in the Toronto protocol, while the LA remains open in the Lund and Hannover strategies. The pulmonary artery cannula is secured with two heavy silk ties proximal to its bifurcation. The trachea is clamped at the level of the carina, and an endotracheal tube is inserted and secured with two heavy silk ties (Fig. 2). A second retrograde flush with 1 L of Perfadex is then performed. The inflated lungs are transferred to the EVLP dome, and the pulmonary artery is connected to the circuit after deairing. In the Toronto protocol, which employs a closed LA system, the LA line is connected. Perfusion is initiated gradually and increased every 10 minutes to reach a steady state, according to the protocol. The endotracheal tube is connected, and ventilation is initiated once the set temperature is reached (Fig. 3). A gas mixture is started after the resumption of ventilation, aiming for a postmembrane partial pressure of CO2 of 35–40 mmHg, with adjustments made to the sweep gas as needed. In the Toronto and Lund protocols, an oxygenator is used to remove oxygen from the perfusate using the gas mixture. In contrast, the Hannover protocol, which utilizes a portable OCS, employs an oxygenator to add oxygen to the perfusate, similar to a conventional cardiopulmonary bypass circuit.
Assessment and evaluation vary depending on the EVLP strategy and are conducted hourly. According to the Toronto protocol, ventilation settings are adjusted to a tidal volume of 10 mL/kg and a rate of 10 breaths per minute, with an FiO2 of 100% for 5 minutes during the evaluation phase. Measurements of pulmonary artery pressure, LA pressure, peak airway pressure, plateau pressure, and both dynamic and static compliance are recorded. Perfusate gas analysis is performed using samples taken from both the venous and arterial sides. A lung X-ray is routinely taken at the 1-hour mark of EVLP and subsequently every 2 hours. In the Lund protocol, an incremental PEEP trial is conducted at 36 °C during the reconditioning phase to aid in lung recruitment. The evaluation commences with the initiation of sweep gas, and blood gas analysis samples are collected from the LA and from the circuit postoxygenator after 10 minutes. The FiO2 is then reduced to 21%, and following a further 10-minute interval, additional blood samples are taken. Finally, the FiO2 is set back to 100% for a third set of blood gas analyses.
The duration of perfusion typically ranges from 2 to 7 hours according to the Lund protocol and may be extended to as long as 12 hours under the Toronto protocol. In the Hannover protocol, perfusion times vary between 3 and 10 hours; this protocol is primarily utilized to prolong ischemia time while maintaining the lungs at warm temperatures. The perfusate solution within the circuit is replaced hourly to compensate for any loss of perfusate and to assist in the reconditioning and repair of the lungs. A decision regarding the suitability of the lungs can generally be made within approximately 2 hours using the Lund technique and within 2 to 3 hours with the Toronto technique. In the case of OCS, once the portable unit arrives at the recipient center, the lungs are assessed and a decision is made immediately (Table 2).
Once the lungs are accepted, perfusion is stopped, and the lungs are ventilated with 50% FiO2. They are then cooled to 10 °C using the Lund strategy and to 15 °C using the Toronto strategy. The inflow and outflow cannulae are clamped and severed. The endotracheal tube is also clamped to keep the lungs inflated. A final antegrade flush is performed with 500 to 1,000 mL of Steen solution. Afterward, the cannulae are removed, and the trachea is sealed with staples. Topical cooling with Perfadex and ice is applied following the same protocol as conventional preservation methods. In the Hannover OCS technique, once the lungs are accepted, the recipient operation continues as usual. A single lung is isolated for ventilation and perfusion within the device, while the contralateral lung remains active. Prior to implantation, each lung is flushed with a cold perfusate [26,29–31].
Stig Steen's successful transplantation of an ex vivo-perfused lung laid the groundwork for the Lund protocol of EVLP. In 2011, Lindstedt et al. [32] compared outcomes between recipients of double lung transplants using standard lungs and those who received transplants following ex vivo perfusion at the University Hospital of Lund during 2006 and 2007.
In the DEVELOP UK multicentric trial, which utilized the Lund protocol for EVLP, the 12-month survival rate for the EVLP group was lower than that of the standard group. However, the data also indicated that there might be no significant difference in survival between the two groups. Additionally, one-third of all organs subjected to EVLP were deemed suitable for transplantation [33].
The first clinical trial of EVLP was reported by Cypel et al. [26] in 2011. The incidence of primary graft dysfunction (PGD) after 72 hours was lower in the EVLP group than in the control group (15% vs. 30.1%), with no cases of severe PGD in the EVLP group at that time point. The rates of bronchial complications requiring intervention were comparable between the two groups. There was no significant difference in the median duration of mechanical ventilation (2 days), nor in the length of intensive care unit (ICU) and hospital stays. The 1-year survival rate was 80% for the EVLP group and 83.6% for the control group.
Slama et al. [34] conducted a prospective randomized trial in 2017 to investigate the role of EVLP in lung transplantation. The study found that the conversion rate of EVLP to transplant was 89.74%. Results indicated that the incidence of PGD greater than grade 1 was lower in the EVLP group compared to the control group (5.7% vs. 19.5%), and the requirement for postoperative extracorporeal membrane oxygenation (ECMO) was also reduced in the EVLP group (5.7% vs. 12.2%). The duration of intubation, as well as ICU and hospital stays, were similar between the two groups. The 30-day survival rate was 97.1% for the EVLP group and 100% for the control group.
In the EXPAND trial conducted by Loor et al. [35] and published in 2019, the use of the OCS for lungs from ECDs and DCD donors was evaluated. The study reported that 87% of the lungs were successfully transplanted following preservation with OCS. Among these, 54% were from ECDs and DCD donors. The incidence of PGD grade 3 (PGD3) within the first 72 hours was 44%. The 30-day posttransplant survival rate was 99%.
Loor et al. [36] evaluated the short- and long-term outcomes of the OCS Lung EXPAND International Trial following lung transplantation with the use of the OCS Lung System. They discovered that there is a high utilization rate of both ECD and DCD donor lungs for transplantation, with similar or improved long-term posttransplant clinical outcomes when compared to those from standard donors.
Along with the physical examination of the donor lung, EVLP allows for bronchoscopic sampling for diagnostic testing, including molecular analysis [37]. Imaging modalities such as lung ultrasound and magnetic resonance imaging can be considered in conjunction with lung X-rays [38,39]. The study of biomarkers and proinflammatory gene expression could aid in assessing the organ's response to ischemia and reperfusion, monitoring inflammation and immune responses, and modifying the immunosuppression protocol [40].
Transmission of infection through a donor organ is an inherent risk in transplantation. The risk factors for infection are higher in EVLP compared to lungs harvested in the standard manner, due to the nature of the inclusion criteria, such as prolonged mechanical ventilation, DCD lungs, and reduced or absent airway reflexes. Infection may impair gas exchange due to cell damage, and the activation of the immune system can also lead to reduced immune tolerance in the recipient. EVLP allows for the targeted treatment of donor lungs in isolation, including the removal of endotoxins, and can permit supratherapeutic drug levels without the associated toxicity to other donor or recipient organs [41–45].
Broad-spectrum antibacterial agents used during EVLP can reduce bacterial load and endotoxin levels, as well as alter proinflammatory markers. This results in a significant improvement in gas exchange and pulmonary mechanics. Interestingly, inhaled nitric oxide, which has been demonstrated to possess antimicrobial properties, has been safely administered in a preclinical model of EVLP. It was used continuously for 12 hours at a concentration of 200 parts per million without the associated risks of side effects such as systemic hypotension and methemoglobinemia [46]. Hepatitis C viral load reduction with ultraviolet C light application (wavelengths of 200– 280 nm) has appeared to be useful for disinfection and reduction of donor transmission of hepatitis C [47].
Although directed immunomodulation therapies such as the administration of the anti-inflammatory agent alpha 1-antitrypsin or the allograft B cell depleting agent rituximab are still under investigation, they are based on the understanding that a proinflammatory state is associated with organ rejection, which in turn is caused by the recipient's immune system recognizing the transplanted organ as foreign [48,49]. The conventional multidrug immunosuppressive protocol can increase the risk of infection and may lead to damage in other end organs. Isolated treatments with cyclosporine and methylprednisolone have demonstrated benefits for physiological parameters and gas exchange during early graft function in preclinical models. Furthermore, therapies using modified adenovirus or lentivirus-based vectors during EVLP can deliver gene therapy directly to the organ, potentially reducing proinflammatory signaling without affecting other organ systems in the recipient [50–53].
Immunomodulation of donor lungs during EVLP has the potential to diminish recognition and sensitization of the recipient's immune system. By increasing tolerance markers, this approach could improve long-term allograft function and recipient survival.
Heart transplantation has progressed since Christiaan Barnard performed the first human heart transplant in 1967, building on the foundational work of Norman Shumway in Cape Town, to become the standard of care [3,54,55]. The donor in that inaugural procedure was a nonheartbeating donor, now referred to as a DCD donor, who had sustained a severe, irreversible brain injury in a road traffic accident and was declared dead following the cessation of cardiac activity after life support was withdrawn in the operating room [17,18]. Barnard's team quickly initiated circulatory support to perfuse and cool the donor's heart before it was harvested for transplantation.
Heart failure carries the highest mortality rate among all organ failures and is associated with a diminished quality of life [56]. Heart transplantation stands as the definitive treatment for heart failure, boasting a median survival period of 10 to 15 years [54,57]. Despite the growing number of transplant centers worldwide, the increasing prevalence of heart failure has resulted in an organ shortage [58]. According to the 2020 Korean Heart Failure White Paper, the estimated prevalence of heart failure in Korea has risen from 0.77% in 2002 to 2.24% in 2018 [59]. The number of heart transplants performed in Korea has climbed to 1,513 cases between 1992 and 2017, with the majority of transplants in Asia being carried out in Taiwan and Korea [60]. Strategies to expand the donor pool include the use of ECDs, improved organ preservation techniques, the utilization of repairable hearts, and the use of DCD donors [61,62].
The heart is traditionally preserved using cold static preservation, also known as static cold storage (SCS), after retrieval. This method may provide an acceptable level of protection for up to 6 hours [63]. Although SCS is considered the gold standard, the donor heart is still subject to time-dependent ischemic and reperfusion injuries, which can impact cardiac function following transplantation. Prolonged cold ischemia time is recognized as a risk factor for primary graft failure and early mortality posttransplant [64,65]. Enhancing organ preservation techniques could expand the donor pool by reducing ischemic injury [62]. Furthermore, renewed interest in DCD, supported by data from animal studies and retrospective studies on pediatric heart transplants, has underscored the need for improvements in organ preservation methods [66,67].
The OCS is currently the sole ex vivo heart perfusion platform that utilizes warm, oxygenated, and nutrient-enriched donor blood to perfuse the coronary arteries [68]. Deoxygenated blood enters the right atrium via the coronary sinus and flows through the tricuspid valve into the right ventricle. It is then ejected through the pulmonary artery to an oxygenator before being returned to the reservoir. The technology is based on the restoration of heart function following the initiation of cardiopulmonary bypass after cardiac arrest, a process known as normothermic regional perfusion [69,70].
The components of the OCS Heart system include the OCS Heart console and the heart perfusion set. The console is an electromechanical, portable device with a wireless monitor that features an infusion pump, a circulatory pump, batteries, a data card, a gas delivery subsystem, and various probes. The heart perfusion set is a single-use, biocompatible device designed for perfusion and monitoring. It consists of a heart perfusion module, a blood collection set, a heart instrumentation tool set, a heart solution set, a cardioplegic arrest set, and a monitoring accessories package. The system requires an internet connection (Wi-Fi) to store, interpret, and compile data.
The perfusate comprises an OCS solution, insulin, antibiotics, methylprednisolone, sodium bicarbonate, multivitamins, and fresh donor blood. A diaphragmatic pump generates pulsatile flow. This system reduces cold ischemia time by keeping the heart in a warm, perfused state during transport, which extends the safe retrieval distance, lowers the incidence of primary graft failure, and expands the donor pool. The OCS system enables continuous monitoring of all heart parameters, including cardiac output, aortic pressure, lactate levels, and coronary blood flow in the graft. Arterial and venous end lactate levels act as surrogate markers for coronary blood flow [71].
•DBD donors: a sternotomy is performed to allow a thorough evaluation of the heart. If deemed suitable for transplantation, the OCS disposable module is unpacked and the machine is assembled. Following complete dissection, a cardioplegia cannula is inserted into the ascending aorta, and a venous drainage cannula is placed in the right atrium. Between 1,200 and 1,500 mL of donor blood is collected from the right atrial cannula into a heparinized bag to prime the OCS machine in preparation for cross-clamping. The standard technique of heart retrieval involves administering Custodiol cardioplegia after the cross-clamp is applied, continuing until the heart is explanted.
•DCD donors: after the administration of 30,000 units of heparin and the withdrawal of life support, if electromechanical arrest occurs within the designated time frame, a prompt sternotomy is performed after a 5-minute wait, and the pericardium is then opened. Between 1,200–1,500 mL of donor blood is collected into a heparinized bag via a large venous cannula placed in the right atrium to prime the OCS machine for the procedure. The subsequent steps for heart retrieval are identical to those used for DBD donors [72].
The heart is prepared for OCS on the back table [73]: (1) Insertion of aortic cannula; an appropriately sized aortic tip cannula is attached to the ascending aorta using a cable tie. This serves as the point of suspension for the heart on the device and provides the route for the delivery of perfusate. (2) Insertion of a pulmonary artery cannula; the pulmonary artery is cannulated using a 30-French straight cannula secured with polypropylene sutures. (3) Insertion of a left ventricular vent to avoid air entrapment and distension of the left ventricle. (4) Closure of the inferior vena cava and superior vena cava along with a patent foramen ovale, so that most of the coronary perfusate is directed from the coronary sinus to the pulmonary artery cannula. (5) Insertion of temporary pacing wires in the ventricular muscle. (6) Placement of defibrillation pads under the atrioventricular groove.
The heart is then attached to the OCS machine using an aortic cannula, and perfusion is initiated with the left ventricle oriented anteriorly. Continuous blood flow is maintained through the aortic cannula to de-air the aorta. The left ventricular vent and pulmonary artery cannula are vented to the cradle of the OCS box, which serves as a reservoir. Coronary perfusion commences once the aortic cannula is connected to the OCS machine at 37 °C, and the heart typically begins to beat within a few minutes. If the heart fibrillates, it is defibrillated. Ventricular pacing wires are placed once the heart achieves sinus rhythm and are set to pace at 80 beats per minute. The heart is sustained with a coronary flow of approximately 700–800 mL/min and an aortic pressure of around 75 mmHg by adjusting the solution rate and aortic blood flow. The heart is covered with a sterile drape for manipulation, if necessary, during transport, and the lid of the tray is closed. Frequent blood gas measurements are taken from both arterial and venous ends to monitor the lactate trend and the levels of potassium and calcium, and left ventricular contractility is periodically evaluated. However, the assessment of the heart's function is limited since the heart is not under load. The heart is paced but not subjected to volume load in either ventricle, which means the ejection fraction cannot be easily determined. In the OCS module, the donor heart rests on the right ventricle and is suspended from the ascending aorta; thus, it is not in the same anatomical position as it is during procurement, which limits direct visualization (Fig. 4).
Upon arrival at the recipient hospital, the heart's contractility and function are reassessed. The decision to proceed with transplantation is based on the heart's function, hemodynamic parameters, and lactate levels (Table 3). If the heart is deemed suitable for transplant, the aortic vent is closed first, and the pulmonary artery cannula is disconnected from the OCS spout. After reducing the OCS flow, the aortic line is clamped, and 1 L of cold Del Nido cardioplegia is administered. Following cardioplegia, the aortic cannula is removed, and the heart is prepared for transplantation [71,72].
OCS may reduce cold ischemic time and extend cardiac graft preservation, which could be particularly beneficial for patients with a history of cardiac surgery (such as congenital heart disease with surgical intervention or previous ventricular assist device implantation), where posttransplant mortality is higher. This approach allows for the safe lysis of all adhesions and preparation for recipient cardiectomy [74,75]. Additionally, it enables the implanting surgeon to evaluate the donor heart's quality before taking any irreversible actions with the recipient. Stamp et al. [76] reported the longest preservation of a donor heart using OCS, achieving an ischemic time of approximately 10.5 hours prior to transplantation in 2015.
The OCS enables the procurement and transportation of hearts over greater distances, thereby increasing the donor pool [77]. The OCS for hearts is particularly useful for evaluating ECD hearts, which may include factors such as donor age over 55 years, decreased left ventricular ejection fraction, left ventricular hypertrophy, history of donor cardiac arrest, anticipated prolonged ischemic time (exceeding 4 hours), history of alcohol or substance abuse, and uncertain coronary artery disease (CAD) status [78]. Additionally, a coronary angiogram can be performed ex vivo to assess for CAD [79]. Elevated aortic pressure and lactate levels serve as surrogate markers for significant CAD when an angiogram is not possible [71]. The OCS system offers the chance to procure marginal hearts and to assess their function in real time after establishing coronary perfusion. If heart function shows improvement, these hearts may be successfully transplanted with favorable outcomes [78]. Looking ahead, the OCS may facilitate potential interventions, such as gene therapy, to alter the immune profile of the graft and decrease the likelihood of rejection [80].
To reduce the deleterious effects of prolonged cold ischemia, the use of the Sherpapak system (Paragonix Technologies) is on the rise for transporting donor hearts. This system preserves the heart within a controlled temperature range of 4 °C to 8 °C in a single-use, sterile disposable box. Utilization of the Sherpapak system has been associated with a lower incidence of severe PGD posttransplant and a decreased need for acute mechanical circulatory support devices. There has been a reduction in the use of ECMO and ventricular assist devices, as well as a decrease in the placement of new intraaortic balloon pumps. Further studies are required to determine whether Sherpapak provides an advantage in terms of prolonged ischemia compared to the OCS [81–84].
Isath et al. [85] conducted a study on ex vivo heart perfusion using the OCS for cases where ischemic time exceeded 4 hours. The study demonstrated the successful utilization of donor hearts from extended distances, which would not have been considered otherwise. Hospital survival rates in the OCS group were 100%, compared to 92.3% in the conventional group. The incidence of PGD was comparable between the two groups, with 12.5% in the OCS group and 15.4% in the conventional group. Notably, no patients in the OCS group required posttransplant venoarterial ECMO support, whereas one patient in the conventional group did [85].
In the first randomized trial to compare DCD heart transplantation with standard criteria DBD (the OCS DCD Heart Trial), there was an overall DCD heart utilization rate of 89%, which demonstrated excellent patient and graft survival outcomes when compared to DBD donor hearts. Patient survival at 6 months was 94.4% for DCD recipients versus 88.6% for the control group, and the graft survival rate at 6 months was 98.9% for DCD recipients compared to 96.7% for the control group. Both patient and graft survival at 1 year were superior in the DCD group compared to the control group. The incidence of moderate or severe ISHLT PGD was 20% in the DCD arm versus 9.1% in the control arm [86].
In the PROCEED II trial, donor hearts demonstrated similar short-term clinical outcomes whether preserved with the OCS or with standard cold storage. The 30-day patient and graft survival rates were 94% in the OCS group and 97% in the control group. Adverse events occurred in 13% of patients in the OCS group and in 14% of patients in the standard cold storage group [87].
Both EVLP and OCS were developed as platforms to evaluate donor graft function and optimize their performance prior to transplantation, with the goal of expanding the donor pool. However, they have yet to achieve widespread use and are currently employed only in major transplant centers.
Multiple studies have demonstrated the feasibility and utility of EVLP since its inception in the early 2000s, despite its resource-intensive nature. A significant initial investment of time and resources is required, including capital for the equipment and hands-on training. Consequently, there is a demand for institutional and national commitment to the growth of lung transplant programs. Despite these challenges, EVLP has proven to be profitable at the institutional level by increasing the volume of transplant programs. Over time, further developments in the field have the potential to revolutionize lung transplantation. EVLP presents a viable pathway to expand the lung donor pool, with the potential to decrease waitlist times and interim mortality. The future of EVLP hinges on the development of therapeutic strategies for infection control and immunomodulation, which could extend the function of allograft lungs.
OCS is the only ex vivo heart perfusion platform that utilizes warm, oxygenated, and enriched donor blood, thereby reducing cold ischemia. This system enables the physiological and metabolic assessment of hearts in a consistent and reproducible manner. OCS offers new opportunities to expand the donor pool by enabling the use of DCD hearts, the procurement of marginal hearts, and the acquisition of hearts from distant locations. Additionally, it offers clinicians more options for assessing donor hearts [89]. OCS introduces innovative approaches that enhance the safety and effectiveness of heart transplantation, particularly in the face of growing surgical complexity. There is potential for OCS to reduce the risk of primary allograft failure, also known as PGD, which is a promising development considering the increasingly complex risk profiles of both donors and recipients in recent years.
Similar to EVLP, OCS requires significant resources and personnel. A clear set of guidelines for the use of both EVLP and OCS, along with a collective effort from government agencies, medical institutions, and clinicians, is urgently needed to ensure the proper allocation of resources and to advance the field of thoracic organ transplantation.
REFERENCES
1. Lund LH, Khush KK, Cherikh WS, Goldfarb S, Kucheryavaya AY, Levvey BJ, et al. 2017; The registry of the International Society for Heart and Lung Transplantation: thirty-fourth adult heart transplantation report-2017; focus theme: allograft ischemic time. J Heart Lung Transplant. 36:1037–46. DOI: 10.1016/j.healun.2017.07.019. PMID: 28779893.

2. Chambers DC, Yusen RD, Cherikh WS, Goldfarb SB, Kucheryavaya AY, Khusch K, et al. 2017; The registry of the International Society for Heart and Lung Transplantation: thirty-fourth adult lung and heart-lung transplantation report-2017; focus theme: allograft ischemic time. J Heart Lung Transplant. 36:1047–59. DOI: 10.1016/j.healun.2017.07.016. PMID: 28784324.
3. Barnard CN. 1967; The operation. A human cardiac transplant: an interim report of a successful operation performed at Groote Schuur Hospital, Cape Town. S Afr Med J. 41:1271–4.
4. Hardy JD, Eraslan S, Webb WR. 1964; Transplantation of the lung. Ann Surg. 160:440–8. DOI: 10.1097/00000658-196409000-00008. PMID: 14206849. PMCID: PMC1408772.

5. Paik HC, Hwang JJ, Kim DH, Joung EK, Kim HK, Lee DY. 2006; The 10 years experience of lung transplantation. Korean J Thorac Cardiovasc Surg. 39:822–7.
6. Korean Network for Organ Sharing (KONOS). 2019. 2019 Annual data report [Internet]. KONOS;Available from: http://konos.go.kr. cited 2023 Dec 23.
7. Jung SH, Kim JJ, Choo SJ, Yun TJ, Chung CH, Lee JW. 2011; Long-term mortality in adult orthotopic heart transplant recipients. J Korean Med Sci. 26:599–603. DOI: 10.3346/jkms.2011.26.5.599. PMID: 21532848. PMCID: PMC3082109.

8. Korea Organ Donation Agency (KODA). 2023. Transplant status: deceased donor organ transplantation status (2000-2022) [Internet]. KODA;Available from: https://www.koda1458.kr/info/transplant.do. cited 2023 Dec 23.
9. Englbrecht JS, Schrader D, Kraus H, Schäfer M, Schedler D, Bach F, et al. 2023; How large is the potential of brain dead donors and what prevents utilization? A multicenter retrospective analysis at seven university hospitals in North Rhine-Westphalia. Transpl Int. 36:11186. DOI: 10.3389/ti.2023.11186. PMID: 37252613. PMCID: PMC10211426.

10. Blackstone EH, Rajeswaran J, Cruz VB, Hsich EM, Koprivanac M, Smedira NG, et al. 2018; Continuously updated estimation of heart transplant waitlist mortality. J Am Coll Cardiol. 72:650–9. DOI: 10.1016/j.jacc.2018.05.045. PMID: 30071995. PMCID: PMC6298792.

11. Valapour M, Lehr CJ, Schladt DP, Smith JM, Goff R, Mupfudze TG, et al. 2023; OPTN/SRTR 2021 annual data report: lung. Am J Transplant. 23(2 Suppl 1):S379–442. DOI: 10.1016/j.ajt.2023.02.009. PMID: 37132345. PMCID: PMC9970343.

12. Yeung JC, Keshavjee S. 2014; Overview of clinical lung transplantation. Cold Spring Harb Perspect Med. 4:a015628. DOI: 10.1101/cshperspect.a015628. PMID: 24384816. PMCID: PMC3869283.

13. Kilic A, Emani S, Sai-Sudhakar CB, Higgins RS, Whitson BA. 2014; Donor selection in heart transplantation. J Thorac Dis. 6:1097–104.
14. Roselli EE, Smedira NG. 2004; Surgical advances in heart and lung transplantation. Anesthesiol Clin North Am. 22:789–807. DOI: 10.1016/j.atc.2004.06.011. PMID: 15541936.

15. Weingarten N, Iyengar A, Herbst DA, Helmers M, Meldrum D, Guevara-Plunkett S, et al. 2023; Extended criteria donor organ use for heart-lung transplantation in the modern era. Clinics (Sao Paulo). 78:100205. DOI: 10.1016/j.clinsp.2023.100205. PMID: 37120982. PMCID: PMC10172855.

16. Van Raemdonck D, Rega F, Rex S, Neyrinck A. 2018; Machine perfusion of thoracic organs. J Thorac Dis. 10(Suppl 8):S910–23. DOI: 10.21037/jtd.2018.02.85. PMID: 29744218. PMCID: PMC5934115.

17. A definition of irreversible coma. 1968; Report of the Ad Hoc Committee of the Harvard Medical School to Examine the Definition of Brain Death. JAMA. 205:337–40. DOI: 10.1001/jama.1968.03140320031009.
18. Kootstra G, Daemen JH, Oomen AP. 1995; Categories of non-heart-beating donors. Transplant Proc. 27:2893–4.
19. Egan TM, Lambert CJ Jr, Reddick R, Ulicny KS Jr, Keagy BA, Wilcox BR. 1991; A strategy to increase the donor pool: use of cadaver lungs for transplantation. Ann Thorac Surg. 52:1113–20. DOI: 10.1016/0003-4975(91)91290-C. PMID: 1953132.

20. Love RB, Stringham J, Chomiak PN, Pellet JR, Mentzer RM. 1995; First successful lung transplantation using a nonheart-beating donor. J Heart Lung Transplant. 14:S88.
21. Ruttens D, Martens A, Ordies S, Verleden SE, Neyrinck AP, Vos R, et al. 2017; Short- and long-term outcomes after lung transplantation from circulatory-dead donors: a single-center experience. Transplantation. 101:2691–4. DOI: 10.1097/TP.0000000000001678. PMID: 28207629.
22. Krutsinger D, Reed RM, Blevins A, Puri V, De Oliveira NC, Zych B, et al. 2015; Lung transplantation from donation after cardiocirculatory death: a systematic review and meta-analysis. J Heart Lung Transplant. 34:675–84. DOI: 10.1016/j.healun.2014.11.009. PMID: 25638297.

23. Cypel M, Levvey B, Van Raemdonck D, Erasmus M, Dark J, Love R, et al. 2015; International Society for Heart and Lung Transplantation Donation After Circulatory Death Registry report. J Heart Lung Transplant. 34:1278–82. DOI: 10.1016/j.healun.2015.08.015. PMID: 26454741.

24. Carrel A, Lindbergh CA. 1935; The culture of whole organs. Science. 81:621–3. DOI: 10.1126/science.81.2112.621. PMID: 17733174.

25. Jirsch DW, Fisk RL, Couves CM. 1970; Ex vivo evaluation of stored lungs. Ann Thorac Surg. 10:163–8. DOI: 10.1016/S0003-4975(10)65582-8. PMID: 4913761.

26. Cypel M, Yeung JC, Liu M, Anraku M, Chen F, Karolak W, et al. 2011; Normothermic ex vivo lung perfusion in clinical lung transplantation. N Engl J Med. 364:1431–40. DOI: 10.1056/NEJMoa1014597. PMID: 21488765.

27. Cypel M, Yeung JC, Machuca T, Chen M, Singer LG, Yasufuku K, et al. 2012; Experience with the first 50 ex vivo lung perfusions in clinical transplantation. J Thorac Cardiovasc Surg. 144:1200–6. DOI: 10.1016/j.jtcvs.2012.08.009. PMID: 22944089.
29. Lindstedt S, Eyjolfsson A, Koul B, Wierup P, Pierre L, Gustafsson R, et al. 2011; How to recondition ex vivo initially rejected donor lungs for clinical transplantation: clinical experience from lund university hospital. J Transplant. 2011:754383. DOI: 10.1155/2011/754383. PMID: 21876780. PMCID: PMC3163037.
30. Warnecke G, Moradiellos J, Tudorache I, Kühn C, Avsar M, Wiegmann B, et al. 2012; Normothermic perfusion of donor lungs for preservation and assessment with the Organ Care System Lung before bilateral transplantation: a pilot study of 12 patients. Lancet. 380:1851–8. DOI: 10.1016/S0140-6736(12)61344-0. PMID: 23063317.

31. Loor G. 2019; EVLP: ready for prime time? Semin Thorac Cardiovasc Surg. 31:1–6. DOI: 10.1053/j.semtcvs.2018.05.005. PMID: 29935227.

32. Lindstedt S, Hlebowicz J, Koul B, Wierup P, Sjögren J, Gustafsson R, et al. 2011; Comparative outcome of double lung transplantation using conventional donor lungs and non-acceptable donor lungs reconditioned ex vivo. Interact Cardiovasc Thorac Surg. 12:162–5. DOI: 10.1510/icvts.2010.244830. PMID: 21123199.

33. Fisher A, Andreasson A, Chrysos A, Lally J, Mamasoula C, Exley C, et al. 2016; An observational study of donor ex vivo lung perfusion in UK lung transplantation: DEVELOP-UK. Health Technol Assess. 20:1–276. DOI: 10.3310/hta20850.

34. Slama A, Schillab L, Barta M, Benedek A, Mitterbauer A, Hoetzenecker K, et al. 2017; Standard donor lung procurement with normothermic ex vivo lung perfusion: a prospective randomized clinical trial. J Heart Lung Transplant. 36:744–53. DOI: 10.1016/j.healun.2017.02.011. PMID: 28314503.

35. Loor G, Warnecke G, Villavicencio MA, Smith MA, Kukreja J, Ardehali A, et al. 2019; Portable normothermic ex-vivo lung perfusion, ventilation, and functional assessment with the Organ Care System on donor lung use for transplantation from extended-criteria donors (EXPAND): a single-arm, pivotal trial. Lancet Respir Med. 7:975–84. DOI: 10.1016/S2213-2600(19)30200-0. PMID: 31378427.

36. Loor G, Warnecke G, Villavicencio MA, Smith MA, Kukreja J, Ardehali A, et al. 2022; Long-term results of the OCS Lung EXPAND international trial using Organ Care System Lung Perfusion System (OCS) in extended-criteria donor (ECD) and donation after circulatory death (DCD) donor lungs. J Heart Lung Transplant. 41(4 Suppl):S43. DOI: 10.1016/j.healun.2022.01.097.

37. Hsin M, Au T. 2018; Ex vivo lung perfusion: a potential platform for molecular diagnosis and ex vivo organ repair. J Thorac Dis. 10(Suppl 16):S1871–83. DOI: 10.21037/jtd.2018.04.119. PMID: 30026974. PMCID: PMC6035934.

38. Renne J, Gutberlet M, Voskrebenzev A, Kern A, Kaireit T, Hinrichs J, et al. 2018; Multiparametric MRI for organ quality assessment in a porcine ex-vivo lung perfusion system. PLoS One. 13:e0209103. DOI: 10.1371/journal.pone.0209103. PMID: 30589907. PMCID: PMC6307703.

39. Ayyat KS, Okamoto T, Niikawa H, Sakanoue I, Dugar S, Latifi SQ, et al. 2020; A CLUE for better assessment of donor lungs: novel technique in clinical ex vivo lung perfusion. J Heart Lung Transplant. 39:1220–7. DOI: 10.1016/j.healun.2020.07.013. PMID: 32773324.

40. Iske J, Hinze CA, Salman J, Haverich A, Tullius SG, Ius F. 2021; The potential of ex vivo lung perfusion on improving organ quality and ameliorating ischemia reperfusion injury. Am J Transplant. 21:3831–9. DOI: 10.1111/ajt.16784. PMID: 34355495. PMCID: PMC8925042.

41. Bunsow E, Los-Arcos I, Martin-Gómez MT, Bello I, Pont T, Berastegui C, et al. 2020; Donor-derived bacterial infections in lung transplant recipients in the era of multidrug resistance. J Infect. 80:190–6. DOI: 10.1016/j.jinf.2019.12.006. PMID: 31843689.

42. Snell G, Hiho S, Levvey B, Sullivan L, Westall G. 2019; Consequences of donor-derived passengers (pathogens, cells, biological molecules and proteins) on clinical outcomes. J Heart Lung Transplant. 38:902–6. DOI: 10.1016/j.healun.2019.06.019. PMID: 31307786.

43. Tanaka S, Gauthier JM, Terada Y, Takahashi T, Li W, Hashimoto K, et al. 2021; Bacterial products in donor airways prevent the induction of lung transplant tolerance. Am J Transplant. 21:353–61. DOI: 10.1111/ajt.16256. PMID: 32786174. PMCID: PMC7775268.

44. Nakajima D, Cypel M, Bonato R, Machuca TN, Iskender I, Hashimoto K, et al. 2016; Ex vivo perfusion treatment of infection in human donor lungs. Am J Transplant. 16:1229–37. DOI: 10.1111/ajt.13562. PMID: 26730551.

45. Zinne N, Krueger M, Hoeltig D, Tuemmler B, Boyle EC, Biancosino C, et al. 2018; Treatment of infected lungs by ex vivo perfusion with high dose antibiotics and autotransplantation: a pilot study in pigs. PLoS One. 13:e0193168. DOI: 10.1371/journal.pone.0193168. PMID: 29505574. PMCID: PMC5837087.

46. Michaelsen VS, Ribeiro RV, Ali A, Wang A, Gazzalle A, Keshavjee S, et al. 2022; Safety of continuous 12-hour delivery of antimicrobial doses of inhaled nitric oxide during ex vivo lung perfusion. J Thorac Cardiovasc Surg. 163:841–9. DOI: 10.1016/j.jtcvs.2020.11.150. PMID: 33478833.
47. Galasso M, Feld JJ, Watanabe Y, Pipkin M, Summers C, Ali A, et al. 2019; Inactivating hepatitis C virus in donor lungs using light therapies during normothermic ex vivo lung perfusion. Nat Commun. 10:481. DOI: 10.1038/s41467-018-08261-z. PMID: 30696822. PMCID: PMC6351537.

48. Lin H, Chen M, Tian F, Tikkanen J, Ding L, Cheung HY, et al. 2018; α1-Anti-trypsin improves function of porcine donor lungs during ex-vivo lung perfusion. J Heart Lung Transplant. 37:656–66. DOI: 10.1016/j.healun.2017.09.019. PMID: 29153638.
49. Ku TJ, Ribeiro RV, Ferreira VH, Galasso M, Keshavjee S, Kumar D, et al. 2020; Ex-vivo delivery of monoclonal antibody (rituximab) to treat human donor lungs prior to transplantation. EBioMedicine. 60:102994. DOI: 10.1016/j.ebiom.2020.102994. PMID: 32950000. PMCID: PMC7501077.

50. Haam S, Noda K, Philips BJ, Harano T, Sanchez PG, Shigemura N. 2020; Cyclosporin A administration during ex vivo lung perfusion preserves lung grafts in rat transplant model. Transplantation. 104:e252–9. DOI: 10.1097/TP.0000000000003237. PMID: 32217944.

51. van Zanden JE, Leuvenink HG, Verschuuren EA, Veldhuis ZJ, Ottens PJ, Erasmus ME, et al. 2021; Ex vivo perfusion with methylprednisolone attenuates brain death-induced lung injury in rats. Transplant Direct. 7:e682. DOI: 10.1097/TXD.0000000000001141. PMID: 33748411. PMCID: PMC7969243.

52. Yeung JC, Wagnetz D, Cypel M, Rubacha M, Koike T, Chun YM, et al. 2012; Ex vivo adenoviral vector gene delivery results in decreased vector-associated inflammation pre- and post-lung transplantation in the pig. Mol Ther. 20:1204–11. DOI: 10.1038/mt.2012.57. PMID: 22453765. PMCID: PMC3369301.

53. Figueiredo C, Carvalho Oliveira M, Chen-Wacker C, Jansson K, Höffler K, Yuzefovych Y, et al. 2019; Immunoengineering of the vascular endothelium to silence MHC expression during normothermic ex vivo lung perfusion. Hum Gene Ther. 30:485–96. DOI: 10.1089/hum.2018.117. PMID: 30261752.
54. Alraies MC, Eckman P. 2014; Adult heart transplant: indications and outcomes. J Thorac Dis. 6:1120–8.
55. Brink JG, Hassoulas J. 2009; The first human heart transplant and further advances in cardiac transplantation at Groote Schuur Hospital and the University of Cape Town - with reference to: the operation. A human cardiac transplant: an interim report of a successful operation performed at Groote Schuur Hospital, Cape Town. Cardiovasc J Afr. 20:31–5.
56. Hessel FP. 2021; Overview of the socio-economic consequences of heart failure. Cardiovasc Diagn Ther. 11:254–62. DOI: 10.21037/cdt-20-291. PMID: 33708497. PMCID: PMC7944217.
57. Lund LH, Edwards LB, Kucheryavaya AY, Dipchand AI, Benden C, Christie JD, et al. 2013; The registry of the International Society for Heart and Lung Transplantation: thirtieth official adult heart transplant report-2013; focus theme: age. J Heart Lung Transplant. 32:951–64. DOI: 10.1016/j.healun.2013.08.006. PMID: 24054804.

58. Javier MF, Javier Delmo EM, Hetzer R. 2021; Evolution of heart transplantation since Barnard's first. Cardiovasc Diagn Ther. 11:171–82. DOI: 10.21037/cdt-20-289. PMID: 33708490. PMCID: PMC7944212.

59. Park JJ, Lee CJ, Park SJ, Choi JO, Choi S, Park SM, et al. 2021; Heart failure statistics in Korea, 2020: a report from the Korean Society of Heart Failure. Int J Heart Fail. 3:224–36. DOI: 10.36628/ijhf.2021.0023. PMID: 36262554. PMCID: PMC9536683.

60. Kim IC, Youn JC, Kobashigawa JA. 2018; The past, present and future of heart transplantation. Korean Circ J. 48:565–90. DOI: 10.4070/kcj.2018.0189. PMID: 29968430. PMCID: PMC6031715.

61. Jacob S, Garg P, Wadiwala I, Yazji JH, Alomari M, Alamouti-fard E, et al. 2022; Strategies for expanding donors pool in heart transplantation. Rev Cardiovasc Med. 23:285. DOI: 10.31083/j.rcm2308285.

62. Hameed AM, Hawthorne WJ, Pleass HC. 2017; Advances in organ preservation for transplantation. ANZ J Surg. 87:976–80. DOI: 10.1111/ans.13713. PMID: 27490874.

63. Hicks M, Hing A, Gao L, Ryan J, Macdonald PS. 2006; Organ preservation. Methods Mol Biol. 333:331–74. DOI: 10.1385/1-59745-049-9:331. PMID: 16790859.

64. Banner NR, Thomas HL, Curnow E, Hussey JC, Rogers CA, Bonser RS, et al. 2008; The importance of cold and warm cardiac ischemia for survival after heart transplantation. Transplantation. 86:542–7. DOI: 10.1097/TP.0b013e31818149b9. PMID: 18724223.

65. Russo MJ, Iribarne A, Hong KN, Ramlawi B, Chen JM, Takayama H, et al. 2010; Factors associated with primary graft failure after heart transplantation. Transplantation. 90:444–50. DOI: 10.1097/TP.0b013e3181e6f1eb. PMID: 20622755.

66. Ali AA, White P, Xiang B, Lin HY, Tsui SS, Ashley E, et al. 2011; Hearts from DCD donors display acceptable biventricular function after heart transplantation in pigs. Am J Transplant. 11:1621–32. DOI: 10.1111/j.1600-6143.2011.03622.x. PMID: 21749639.

67. Boucek MM, Kanakriyeh MS, Mathis CM, Trimm RF 3rd, Bailey LL. 1990; Cardiac transplantation in infancy: donors and recipients. J Pediatr. 116:171–6. DOI: 10.1016/S0022-3476(05)82870-7. PMID: 2299486.

68. Pahuja M, Case BC, Molina EJ, Waksman R. 2022; Overview of the FDA's Circulatory System Devices Panel virtual meeting on the TransMedics Organ Care System (OCS) Heart - portable extracorporeal heart perfusion and monitoring system. Am Heart J. 247:90–9. DOI: 10.1016/j.ahj.2022.02.003. PMID: 35150637.

69. Fedalen PA, Piacentino V, Popovich DA. 2000; Protection and resuscitation of non-beating hearts: a potential approach to increase the donor pool. J Heart Lung Transplant. 19:97.
70. Hassanein WH, Zellos L, Tyrrell TA, Healey NA, Crittenden MD, Birjiniuk V, et al. 1998; Continuous perfusion of donor hearts in the beating state extends preservation time and improves recovery of function. J Thorac Cardiovasc Surg. 116:821–30. DOI: 10.1016/S0022-5223(98)00452-8. PMID: 9806389.

71. García Sáez D, Zych B, Sabashnikov A, Bowles CT, De Robertis F, Mohite PN, et al. 2014; Evaluation of the organ care system in heart transplantation with an adverse donor/recipient profile. Ann Thorac Surg. 98:2099–105. DOI: 10.1016/j.athoracsur.2014.06.098. PMID: 25443013.

72. Mohite P, Husain M, Saez DG, Penn S, Maunz O, Simon A. 2020. Utilization of TransMedics organ care system for preservation of the donor heart [Internet]. CTSNeT;Available from: https://doi.org/10.25373/ctsnet.13374056. cited 2023 Dec 23. DOI: 10.25373/ctsnet.13374056.

73. Bryner BS, Schroder JN, Milano CA. 2021; Heart transplant advances: ex vivo organ-preservation systems. JTCVS Open. 8:123–7. DOI: 10.1016/j.xjon.2021.04.020. PMID: 36004090. PMCID: PMC9390583.

74. Rojas SV, Avsar M, Ius F, Schibilsky D, Kaufeld T, Benk C, et al. 2022; Ex-vivo preservation with the organ care system in high risk heart transplantation. Life (Basel). 12:247. DOI: 10.3390/life12020247. PMID: 35207534. PMCID: PMC8877453.

75. Beuth J, Falter F, Pinto Ribeiro RV, Badiwala M, Meineri M. 2019; New strategies to expand and optimize heart donor pool: ex vivo heart perfusion and donation after circulatory death: a review of current research and future trends. Anesth Analg. 128:406–13. DOI: 10.1213/ANE.0000000000003919. PMID: 30531220.

76. Stamp NL, Shah A, Vincent V, Wright B, Wood C, Pavey W, et al. 2015; Successful heart transplant after ten hours out-of-body time using the TransMedics Organ Care System. Heart Lung Circ. 24:611–3. DOI: 10.1016/j.hlc.2015.01.005. PMID: 25697385.

77. Pinnelas R, Kobashigawa JA. 2022; Ex vivo normothermic perfusion in heart transplantation: a review of the TransMedics® Organ Care System. Future Cardiol. 18:5–15. DOI: 10.2217/fca-2021-0030. PMID: 34503344.

78. White CW, Messer SJ, Large SR, Conway J, Kim DH, Kutsogiannis DJ, et al. 2018; Transplantation of hearts donated after circulatory death. Front Cardiovasc Med. 5:8. DOI: 10.3389/fcvm.2018.00008. PMID: 29487855. PMCID: PMC5816942.

79. Ghodsizad A, Bordel V, Ungerer M, Karck M, Bekeredjian R, Ruhparwar A. 2012; Ex vivo coronary angiography of a donor heart in the organ care system. Heart Surg Forum. 15:E161–3. DOI: 10.1532/HSF98.20111146. PMID: 22698606.

80. Bishawi M, Roan JN, Milano CA, Daneshmand MA, Schroder JN, Chiang Y, et al. 2019; A normothermic ex vivo organ perfusion delivery method for cardiac transplantation gene therapy. Sci Rep. 9:8029. DOI: 10.1038/s41598-019-43737-y. PMID: 31142753. PMCID: PMC6541710.

81. Radakovic D, Karimli S, Penov K, Schade I, Hamouda K, Bening C, et al. 2020; First clinical experience with the novel cold storage SherpaPak™ system for donor heart transportation. J Thorac Dis. 12:7227–35. DOI: 10.21037/jtd-20-1827. PMID: 33447411. PMCID: PMC7797872.

82. D'Alessandro D, Philpott J, Boeve T, Pham S, Zuckermann A. 2021; First report of the GUARDIAN Registry: an international consortium examining the effect of controlled hypothermic preservation in heart transplantation. J Heart Lung Transplant. 40(4 Suppl):S127. DOI: 10.1016/j.healun.2021.01.398.
83. Leacche M, Philpott J, Pham S, Shudo Y, Kawabori M, Jacobs J, et al. 2022; US multi-center analysis of the Global Utilization and Registry Database for Improved Heart Preservation (GUARDIAN) registry: 1-year transplant survival analysis. J Heart Lung Transplant. 41(4 Suppl):S30–1. DOI: 10.1016/j.healun.2022.01.067.

84. Zuckermann A, Leacche M, Philpott J, Pham S, Shudo Y, Bustamante-Munguira J, et al. 2022; Second report of the GUARDIAN registry: an international consortium examining effect of controlled hypothermic preservation in heart transplantation. J Heart Lung Transplant. 41(4 Suppl):S477. DOI: 10.1016/j.healun.2022.01.1204.
85. Isath A, Ohira S, Levine A, Pan S, Aggarwal-Gupta C, Lanier GM, et al. 2023; Ex vivo heart perfusion for cardiac transplantation allowing for prolonged perfusion time and extension of distance traveled for procurement of donor hearts: an initial experience in the United States. Transplant Direct. 9:e1455. DOI: 10.1097/TXD.0000000000001455. PMID: 36845853. PMCID: PMC9949869.

86. Schroder JN, Shah A, Pretorius V, Smith J, Daneshmand M, Geirsson A, et al. 2022; Expanding heart transplants from donors after circulatory death (DCD) - results of the first randomized controlled trial using the Organ Care System (OCS™) heart - (OCS DCD heart trial). J Heart Lung Transplant. 41(4 Suppl):S72. DOI: 10.1016/j.healun.2022.01.165.

87. Ardehali A, Esmailian F, Deng M, Soltesz E, Hsich E, Naka Y, et al. 2015; Ex-vivo perfusion of donor hearts for human heart transplantation (PROCEED II): a prospective, open-label, multicentre, randomised non-inferiority trial. Lancet. 385:2577–84. DOI: 10.1016/S0140-6736(15)60261-6. PMID: 25888086.

Fig. 1
Schematic representation of an ex vivo lung perfusion (EVLP) circuit. PA, pulmonary artery; LA, left atrium.
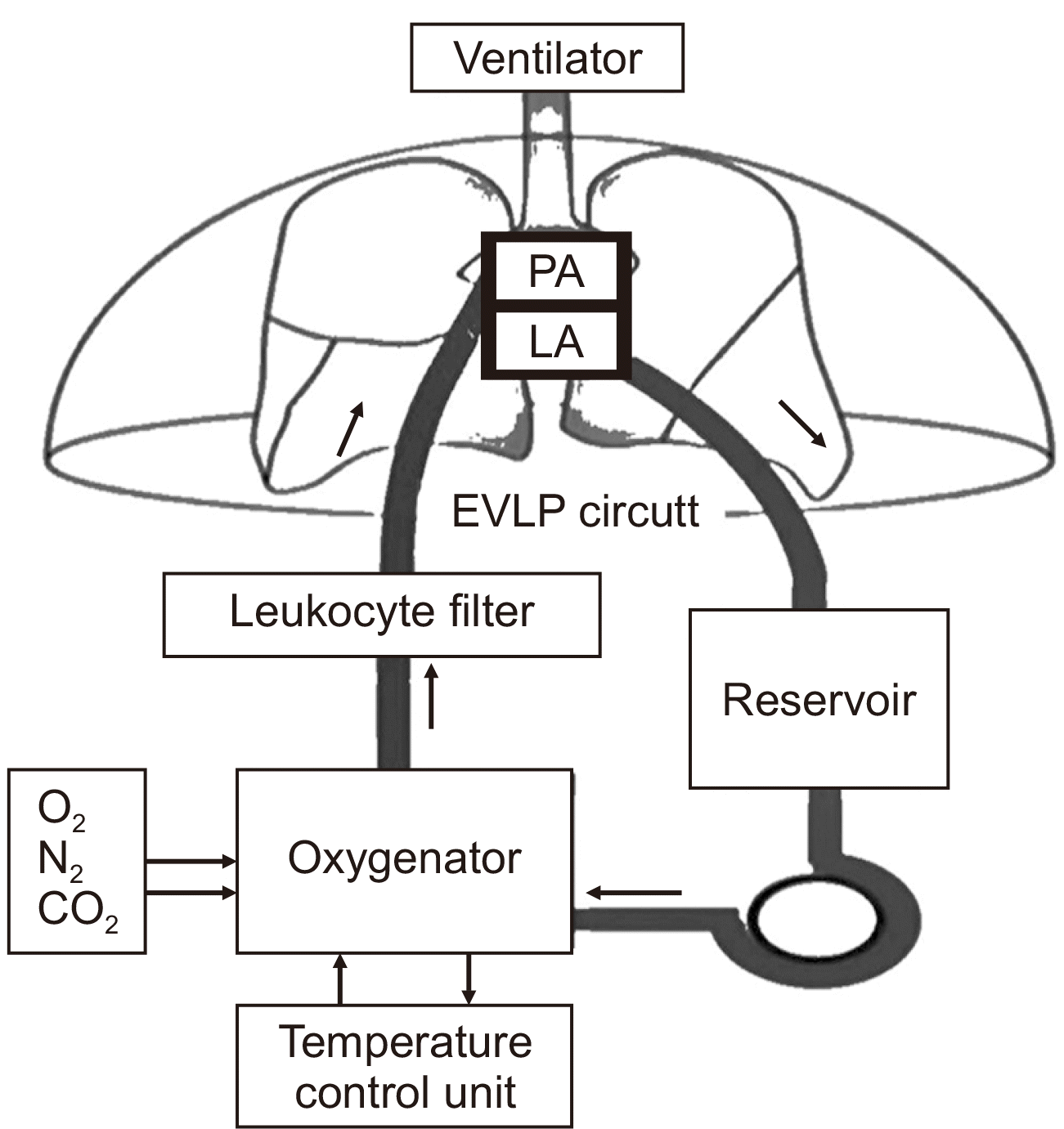
Fig. 2
Ex vivo lung perfusion cannulation: green cannula in the left atrium, yellow cannula in the pulmonary artery, and endotracheal tube in trachea.
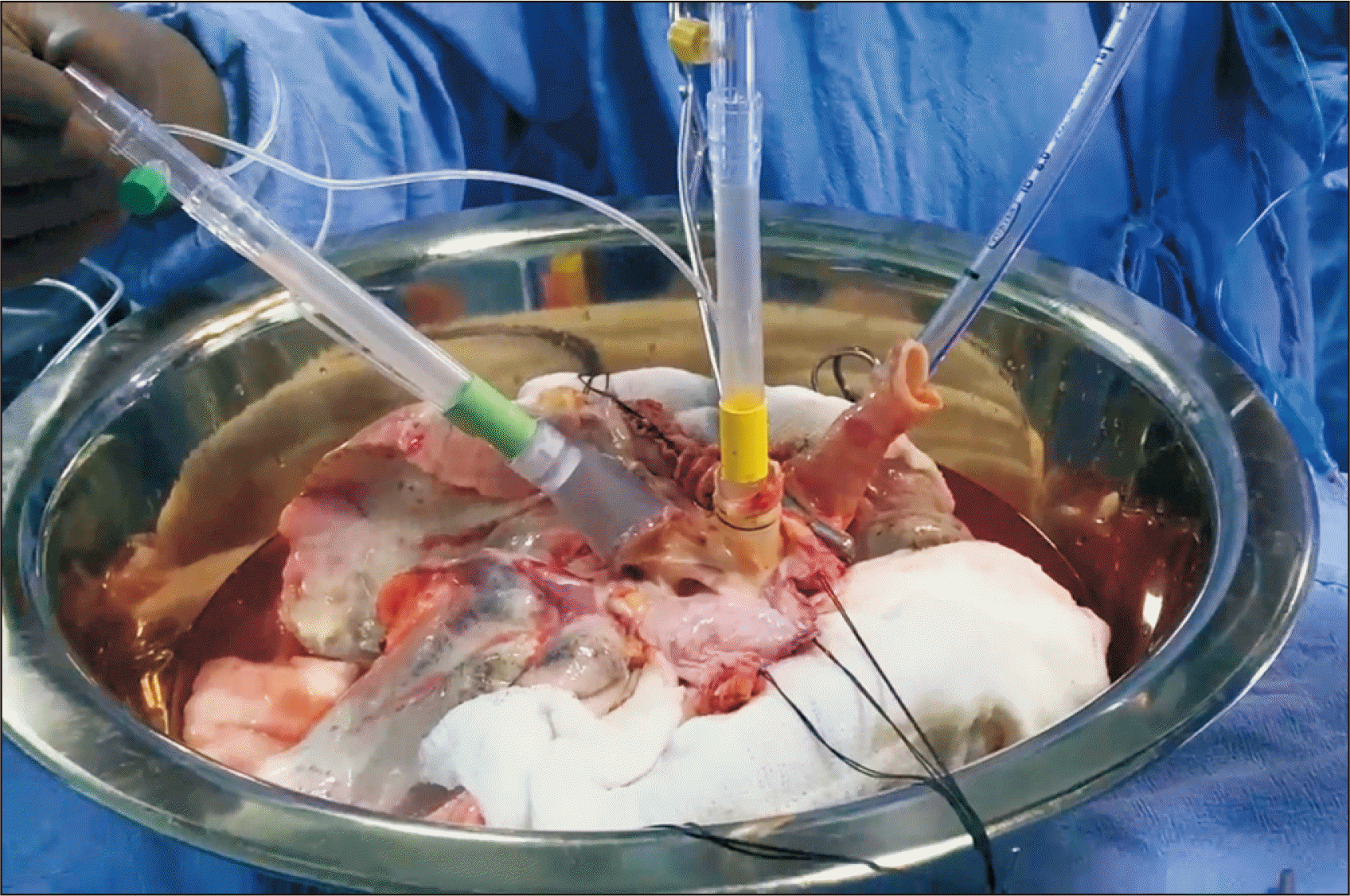
Fig. 3
Manual ex vivo lung perfusion circuit. The lung within the dome is within the in-line sensors.

Fig. 4
Schematic diagram of an Organ Care System circuit. AO, aorta; PA, pulmonary artery; LV, left ventricle; RV, right ventricle; LA, left atrium.
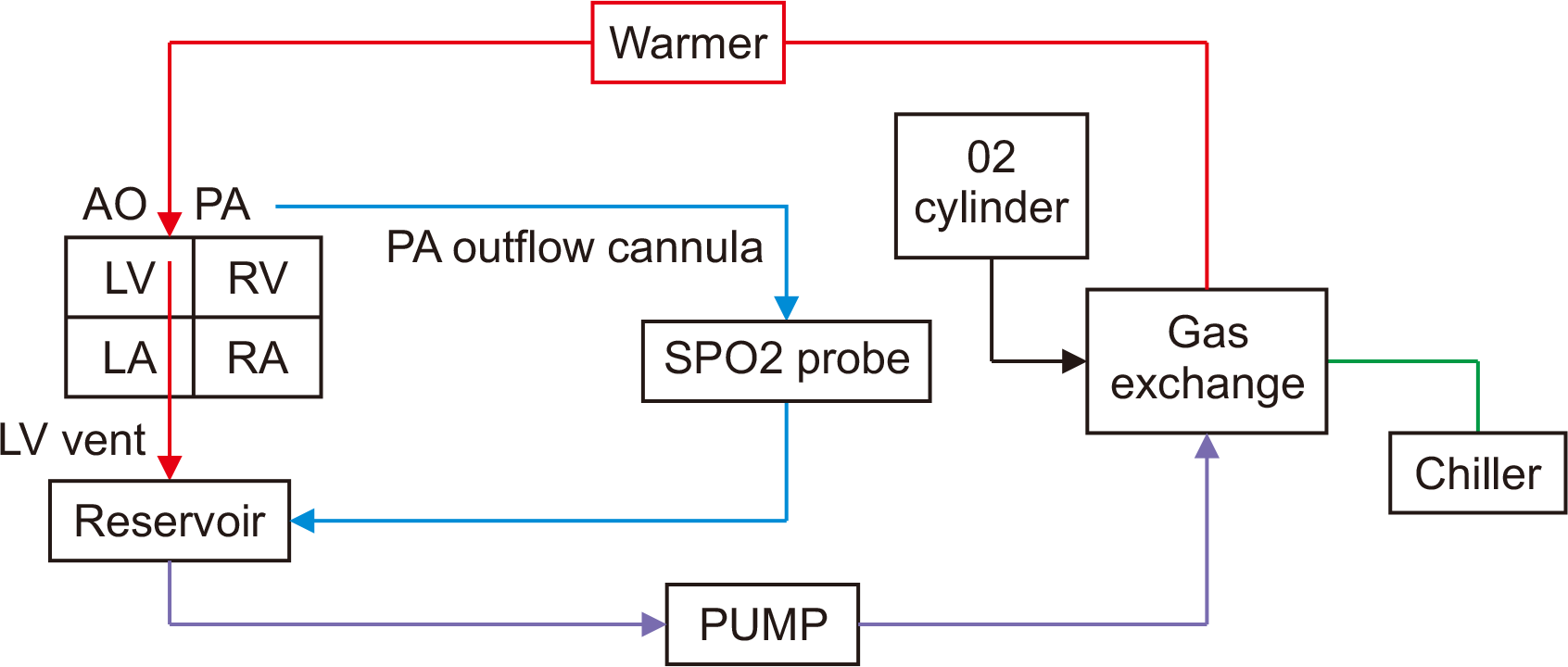
Table 1
Various ex vivo lung perfusion protocols
Table 2
Criteria for the acceptance of lungs on ex vivo lung perfusion
Table 3
Allograft assessment on OCS




 PDF
PDF Citation
Citation Print
Print



 XML Download
XML Download