Abstract
Despite the increasing demand for lung transplants, donor lungs remain in short supply. Although organ donations have been steadily increasing in Korea, with the utilization rate for donor lungs increasing to 40% in recent years, many potential donor organs remain unused. To match the increasing number of patients on the lung transplant waitlist, it is essential to increase the donor procurement rate through optimal management. Improvements in donor lung management programs can lead to expansion of the donor pool and optimal posttransplant outcomes. This review focuses on basic protocols for the optimal management of donor lungs and summarizes coronavirus disease 2019-related considerations for donor lung evaluation.
Over the past few decades, lung transplantation has emerged as a viable treatment option for end-stage lung disease, and the number of lung transplantation procedures worldwide is rapidly increasing [1]. While the demand for lung transplantation in Korea continues to rise, the number of deaths while awaiting transplantation remains high due to the lack of donors [2,3]. Proper donor management is critical for improving organ function and maximizing the procurement rate from the available donor pool. Physicians caring for potentially brain-dead individuals should conduct thorough evaluations with an eye toward the possibility of donation. If an individual is willing to donate, proactive care must be provided to optimize the organ status. This review provides a current update on appropriate donor management from the perspective of the lung transplant team.
In Korea, most organs come from patients who have been certified as brain-dead. Severe brain injury can result in neurogenic pulmonary edema [4]. This condition arises from elevated pulmonary hydrostatic pressure caused by a sudden surge in circulating catecholamines and increased expression of inflammatory mediators, leading to heightened lung capillary permeability and interstitial edema. Furthermore, brain-dead donors face an elevated risk of aspiration, infection, and mechanical ventilation-related injury. As a result, very few lungs from these donors are deemed suitable for transplantation. According to data from the Korea Network for Organ Sharing (KONOS), lungs were procured and transplanted from 12% of the brain-dead donor population [5].
Primary graft dysfunction (PGD) is a severe form of lung injury occurring within the first 72 hours after lung transplantation, and it is the most common cause of early mortality [6]. The donor's age, smoking history, chronic alcohol use, lung size, aspiration, chest trauma, lung contusion, history of traumatic brain injury, time from brain death to cold preservation, and mechanical ventilation time may all be associated with PGD [7]. Therefore, appropriate donor lung selection and optimal preservation are essential for reducing the risk of PGD. Above all, it is crucial to minimize damage to the donor's lungs through appropriate management of the donor.
The goals of managing donation after brain death (DBD) include optimizing cardiac filling pressures, maintaining adequate arterial pressure for donor organ perfusion, ensuring an open airway, maintaining protective ventilation strategies, and maintaining metabolic homeostasis [8]. The International Society for Heart and Lung Transplantation (ISHLT) guidance for managing DBD donors is summarized in Fig. 1.
The hemodynamic status of the donor should be continuously monitored via arterial and central venous lines, and pulmonary artery catheters should be considered for donors with low mean arterial pressure (<60 mmHg) despite adequate central venous pressure (CVP; 6–10 mmHg). Potential lung donors require continuous oxygen saturation and end-tidal carbon dioxide monitoring at regular intervals (at least daily), arterial blood gases, and daily chest radiographs and computed tomography (CT) scans. A PaO2:FiO2 (P/F) ratio >300 mmHg generally indicates that the lungs are suitable for transplantation, although a lower value does not necessarily indicate unsuitability for donation. Active use of diuretics, recruitment maneuvers, removal of secretions, and management of atelectasis through prone positioning can help improve the P/F ratio.
Many organ donors face the potential for intravascular volume depletion due to diminished vascular tone and heightened capillary permeability. Diabetes insipidus (DI) and hyperglycemia may further exacerbate these losses, worsening the susceptibility to hypovolemic shock. However, because massive crystalloid infusions can cause pulmonary edema and harm arterial oxygenation, a conservative fluid approach is required [9]. Approximately 90% of brain-dead donors need vasopressor support despite adequate fluid resuscitation. Vasopressin has recently emerged as a recommended first-line agent. This agonist, acting on V1 and V2 receptors, prevents DI while increasing systemic vascular resistance. It has been linked to improved donor heart function and increased recovery rate of donor organs [10].
Hormone therapy after brain death in combination with a CVP <10 mmHg significantly improves the utilization of the lungs for transplantation without affecting other organ systems [11]. Achieving a final CVP <10 mmHg enabled the transplantation of 44% more hearts, 95% more lungs, and 13% more kidneys. High-dose corticosteroid administration also improved oxygenation and increased lung procurement success compared with controls [12]. A recent study found that a low dose of hydrocortisone (300 mg) achieved similar improvements in hemodynamic stability and pulmonary oxygenation while causing less hyperglycemia compared with high-dose methylprednisolone [13]. In a review by Callahan et al. [14], lung function was preserved to a greater degree in donors receiving arginine vasopressin (AVP). When adjusting for significant factors, AVP was independently associated with lung procurement (odds ratio, 1.220; 95% confidence interval, 1.114–1.336; P<0.001). Recommended hormone regimens are shown in Table 1.
To prevent aspiration, the head of the bed should be elevated to 30°, and the endotracheal tube balloon should be inflated to a pressure of 25 cmH2O [9]. A protective ventilation strategy plays a crucial role in the respiratory management of potential lung donors, helping to reduce the risk of ventilator-induced lung injury [8]. Lung-protective ventilation protocols have demonstrated significant benefits compared to conventional strategies in randomized controlled trials, including increased donor eligibility (95% vs. 54%) and a more significant number of lungs suitable for transplantation (54% vs. 27%) [15].
After tracheal suction or temporary disconnection of the ventilator circuit, gentle recruitment maneuvers should be performed. Additionally, a PaO2 of less than 500 mmHg is recommended to reduce the risk of subsequent bronchiolitis obliterans in lung recipients. After completion of the evaluation, FiO2 should be reduced immediately to avoid unintentional collateral damage to the lungs. If the patient is dependent on positive-end expiratory pressure (PEEP), tracheal suctioning should be performed only in the presence of airway secretions to avoid disruption of recruitment.
Bronchoscopy is conducted promptly to ensure a precise assessment of conditions such as bronchitis and aspiration and to obtain sputum samples or perform bronchoalveolar lavage when an infection is suspected [8]. This procedure may also be employed to remove stagnant secretions that could potentially lead to atelectasis or inhalation injury. Higher PEEP combined with recruitment maneuvers or prone positioning may help reduce ventilation-perfusion mismatching and diffusion abnormalities, thereby increasing the P/F ratio [16]. Notably, the University Hospital Marqués de Valdecilla in Spain implemented a strict donor management protocol that significantly improved the lung utilization rate from 20% to 50% [17] (Table 2). Implementing an intensive lung donor-management protocol based on synergic measures increases the lung procurement rate.
Managing donor lungs in the context of coronavirus disease 2019 (COVID-19) requires careful consideration of the following factors. (A) If the donor has been exposed to a confirmed or suspected case of COVID-19 within the past 10 days: the organ may be considered for cardiothoracic transplant if the donor has been asymptomatic for more than 7 days since exposure and there is at least one negative severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) polymerase chain reaction (PCR) test along with a chest CT scan negative for pulmonary infection. (B) If the donor has a history of confirmed COVID-19, they may be considered for transplantation under the following conditions: clinical resolution of COVID-19 symptoms, the passage of over 21 days from the onset of symptoms in an immunocompetent donor, no significant pulmonary disease due to COVID-19, and at least one negative SARS-CoV-2 PCR test, along with a chest CT scan showing no signs of pulmonary infection/chronic lung injury.
Globally, donation after cardiac death (DCD) donor lungs have significantly increased lung transplant activity, shortened time spent on the waitlist, and decreased waitlist mortality rates [18]. The outcomes of lung transplantation using DCD donors have shown no significant differences compared to those using DBD donors. With these positive results, legislation concerning DCD is currently being promoted in Korea. In the future, specifically considering donor management strategies related to DCD will also be necessary to ensure the active implementation and efficient use of DCD. In addition, researchers' interest in this field is necessary, as innovative studies such as ex vivo lung perfusion and lung preservation at 10 °C are underway [19,20].
REFERENCES
1. Valapour M, Lehr CJ, Schladt DP, Smith JM, Goff R, Mupfudze TG, et al. 2023; OPTN/SRTR 2021 annual data report: lung. Am J Transplant. 23(2 Suppl 1):S379–442. DOI: 10.1016/j.ajt.2023.02.009. PMID: 37132345. PMCID: PMC9970343.

2. Yeo HJ, Oh DK, Yu WS, Choi SM, Jeon K, Ha M, et al. 2022; Outcomes of patients on the lung transplantation waitlist in Korea: a Korean Network for Organ Sharing data analysis. J Korean Med Sci. 37:e294. DOI: 10.3346/jkms.2022.37.e294. PMID: 36281485. PMCID: PMC9592937.

3. Yeo HJ, Kim DH, Kim YS, Jeon D, Cho WH. 2021; Performance changes following the revision of organ allocation system of lung transplant: analysis of Korean Network for Organ Sharing data. J Korean Med Sci. 36:e79. DOI: 10.3346/jkms.2021.36.e79. PMID: 33783144. PMCID: PMC8007421.

4. Busl KM, Bleck TP. 2015; Neurogenic pulmonary edema. Crit Care Med. 43:1710–5. DOI: 10.1097/CCM.0000000000001101. PMID: 26066018.

5. Yeo HJ, Yoon SH, Lee SE, Jeon D, Kim YS, Cho WH, et al. 2017; Current status and future of lung donation in Korea. J Korean Med Sci. 32:1953–8. DOI: 10.3346/jkms.2017.32.12.1953. PMID: 29115076. PMCID: PMC5680493.

6. Snell GI, Yusen RD, Weill D, Strueber M, Garrity E, Reed A, et al. 2017; Report of the ISHLT working group on primary lung graft dysfunction, part I: definition and grading: a 2016 consensus group statement of the International Society for Heart and Lung Transplantation. J Heart Lung Transplant. 36:1097–103. DOI: 10.1016/j.healun.2017.07.021. PMID: 28942784.
7. Venkata-Subramani M, Nunley DR, Roman J. 2021; Donor factors and risk of primary graft dysfunction and mortality post lung transplantation: a proposed conceptual framework. Clin Transplant. 35:e14480. DOI: 10.1111/ctr.14480. PMID: 34516007.

8. Copeland H, Hayanga JW, Neyrinck A, MacDonald P, Dellgren G, Bertolotti A, et al. 2020; Donor heart and lung procurement: a consensus statement. J Heart Lung Transplant. 39:501–17. DOI: 10.1016/j.healun.2020.03.020. PMID: 32503726.

9. Angel LF, Levine DJ, Restrepo MI, Johnson S, Sako E, Carpenter A, et al. 2006; Impact of a lung transplantation donor-management protocol on lung donation and recipient outcomes. Am J Respir Crit Care Med. 174:710–6. DOI: 10.1164/rccm.200603-432OC. PMID: 16799075.

10. Plurad DS, Bricker S, Neville A, Bongard F, Putnam B. 2012; Arginine vasopressin significantly increases the rate of successful organ procurement in potential donors. Am J Surg. 204:856–60. DOI: 10.1016/j.amjsurg.2012.05.011. PMID: 23116641.

11. Kumar L. 2016; Brain death and care of the organ donor. J Anaesthesiol Cli Pharmacol. 32:146–52. DOI: 10.4103/0970-9185.168266. PMID: 27275040. PMCID: PMC4874065.

12. Follette DM, Rudich SM, Babcock WD. 1998; Improved oxygenation and increased lung donor recovery with high-dose steroid administration after brain death. J Heart Lung Transplant. 17:423–9.
13. Dhar R, Cotton C, Coleman J, Brockmeier D, Kappel D, Marklin G, et al. 2013; Comparison of high- and low-dose corticosteroid regimens for organ donor management. J Crit Care. 28:111.e1–7. DOI: 10.1016/j.jcrc.2012.04.015. PMID: 22762934.

14. Callahan DS, Neville A, Bricker S, Kim D, Putnam B, Bongard F, et al. 2014; The effect of arginine vasopressin on organ donor procurement and lung function. J Surg Res. 186:452–7. DOI: 10.1016/j.jss.2013.09.028. PMID: 24176209.

15. Mascia L, Pasero D, Slutsky AS, Arguis MJ, Berardino M, Grasso S, et al. 2010; Effect of a lung protective strategy for organ donors on eligibility and availability of lungs for transplantation: a randomized controlled trial. JAMA. 304:2620–7. DOI: 10.1001/jama.2010.1796. PMID: 21156950.

16. Son E, Jang J, Cho WH, Kim D, Yeo HJ. 2021; Successful lung transplantation after prone positioning in an ineligible donor: a case report. Gen Thorac Cardiovasc Surg. 69:1352–5. DOI: 10.1007/s11748-021-01676-4. PMID: 34159516. PMCID: PMC8218964.

17. Miñambres E, Coll E, Duerto J, Suberviola B, Mons R, Cifrian JM, et al. 2014; Effect of an intensive lung donor-management protocol on lung transplantation outcomes. J Heart Lung Transplant. 33:178–84. DOI: 10.1016/j.healun.2013.10.034. PMID: 24365763.

18. Bobba CM, Whitson BA, Henn MC, Mokadam NA, Keller BC, Rosenheck J, et al. 2022; Trends in donation after circulatory death in lung transplantation in the United States: impact of era. Transpl Int. 35:10172. DOI: 10.3389/ti.2022.10172. PMID: 35444490. PMCID: PMC9013720.

19. Nakajima D, Date H. 2021; Ex vivo lung perfusion in lung transplantation. Gen Thorac Cardiovasc Surg. 69:625–30. DOI: 10.1007/s11748-021-01609-1. PMID: 33683575. PMCID: PMC7938286.

20. Ali A, Wang A, Ribeiro RV, Beroncal EL, Baciu C, Galasso M, et al. 2021; Static lung storage at 10°C maintains mitochondrial health and preserves donor organ function. Sci Transl Med. 13:eabf7601. DOI: 10.1126/scitranslmed.abf7601. PMID: 34524862.

Fig. 1
Donor heart and lung management algorithm. ECG, electrocardiogram; CXR, chest X-ray; ABG, arterial blood gas; AFB's, acid fast fluorescent stain; CVP, central venous pressure; MAP, mean arterial pressure; TV, tidal volume; PEEP, positive-end expiratory pressure; IV, intravenous; BGL, blood glucose level; Echo, echocardiogram; T3, tri-iodothyronine; T4, thyroxine; LV, left ventricle; LVEF, left ventricular ejection fraction. Reproduced from Copeland et al. [8] with permission of Elsevier according to the Creative Commons license.
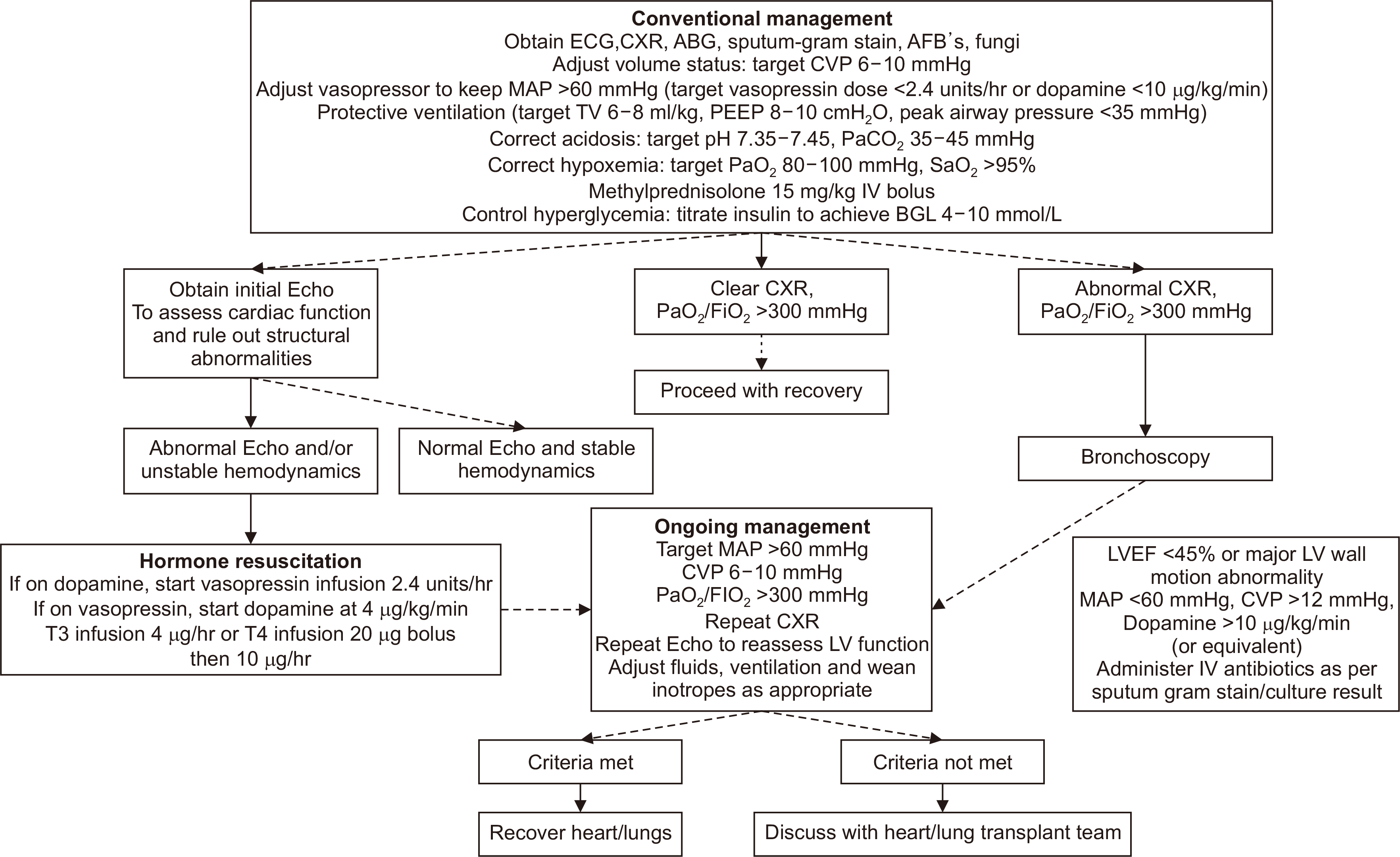
Table 1
Hormone replacement therapy
Table 2
Lung donor-management protocol at University Hospital Marqués de Valdecilla




 PDF
PDF Citation
Citation Print
Print



 XML Download
XML Download