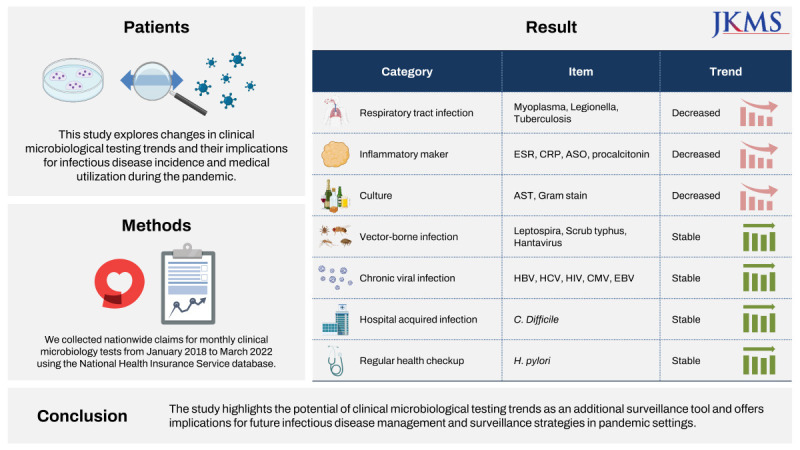
Abstract
The coronavirus disease 2019 pandemic has brought significant changes to infectious disease management globally. This study explored changes in clinical microbiological testing trends and their implications for infectious disease incidence and medical utilization during the pandemic. We collected nationwide claims for monthly clinical microbiology tests from January 2018 to March 2022 using the National Health Insurance Service database. Seasonal autoregressive integrated moving average models were employed to make predictions for each disease based on the baseline period (January 2018 to January 2020). The results showed a significant decrease in general bacterial and fungal cultures, respiratory infectious disease-related, and inflammatory markers, while the representatives of tests for vector-borne diseases, healthcare-associated infections, and chronic viral infections remained stable. The study highlights the potential of clinical microbiological testing trends as an additional surveillance tool and offers implications for future infectious disease management and surveillance strategies in pandemic settings.
Graphical Abstract
The coronavirus disease 2019 (COVID-19) pandemic has had a substantial impact on the approach to infectious disease management worldwide.1 In the first two years of the pandemic, non-pharmacological interventions (NPIs), including mandatory mask-wearing, social distancing, contact tracing, and large-scale diagnostic testing, were implemented to reduce the transmission of severe acute respiratory syndrome-coronavirus-2.23 While these policies were effective in controlling the spread of COVID-19, they also resulted in changes in the incidence and management of other infectious diseases.4 Notifiable diseases, such as influenza, have seen a decrease in incidence,56 and the spread of traditional infectious diseases, such as chickenpox and mumps, has been suppressed.78 These changes have primarily been observed through national notifiable diseases surveillance systems and reporting of clinical data. However, whether clinical microbiological testing patterns required at the national level reflect the changes in the incidence and management of various infectious diseases, remains unknown.
In this study, we aimed to investigate whether changes in clinical microbiological testing trends provide insights into the changes in the incidence of and medical utilization for infectious diseases during the COVID-19 pandemic. We used South Korean nationwide health insurance claims data to analyze the trends in clinical microbiological tests during the COVID-19 period. The results of this study may have implications for the future management and surveillance of infectious diseases in a pandemic setting.
The number of nationwide claims related to monthly clinical microbiology tests for January 2018 to March 2022 was collected from the database of the National Health Insurance Service (NHIS). In South Korea, 607 infectious disease-related diagnostic tests are covered by national health insurance. The Electronic Data Interchange medical procedure code of the diagnostic test corresponds to the Current Procedural Terminology code used in the United States. We tracked the changes before and after COVID-19 in all diagnostic tests and presented those with significant changes or meaningful trends, here. Using a seasonal autoregressive integrated moving average (SARIMA), prediction models were created for each disease based on the incidence across the entire baseline period. The baseline period was defined as January 2018 to January 2020, when COVID-19 was first confirmed in South Korea. The parameters for the SARIMA models were chosen using the “auto.arima” function in the R software package, “forecast” (https://pkg.robjhyndman.com/forecast/). In addition, the parameters were determined using the corrected Akaike information criterion complementary to the auto.arima function. The 95% confidence interval of the projected values and the monthly records of different types of infectious disease-related diagnostic tests during the intervention period were visually compared.
We selected clinical microbiological tests related to infection and inflammation in the NHIS database. Among a total of 607 diagnostic tests, 360 tests showed a stable number of claims suitable for time series analysis. We focused our presentation on a total of 21 tests that exhibited the most significant or meaningful trends (Fig. 1, Table 1). These were classified into seven categories: respiratory infection tests, inflammatory markers, culture tests, vector-borne disease tests, chronic viral infection tests, nosocomial infection-related tests, and health check-up-related tests. Fig. 1 illustrates the monthly fluctuations in the number of tests conducted before and after the onset of COVID-19, including a two-year forecast generated under the assumption of the absence of COVID-19. These projections were based on the data collected two years prior to the pandemic. In order to account for discrepancies in the actual and predicted values, we employed a time-series ARIMA model. This allowed us to visually compare the data and particularly assess for significant deviations. Given that it can be challenging to claim a distinct decrease for diseases such as vector-borne infections based on visual examination alone, we supplemented this approach with traditional statistical testing methods. Table 1, on the other hand, compares the average monthly changes in the number of tests performed before and after the advent of COVID-19. However, it is worth noting that due to the inherent limitations of monthly measurements, statistical power is unavoidably diminished, and the P values derived from t-tests used here may differ from the confidence intervals presented in the time-series model. Results of the remaining diagnostic tests are available in Supplementary Table 1 and Supplementary Fig. 1.
The results revealed notable changes in the testing patterns for various infectious diseases since the onset of the COVID-19 pandemic. A significant decrease was observed in respiratory infection testing, including tests for Mycoplasma, Legionella, and tuberculosis. Inflammatory marker tests, such as those for C-reactive protein and the erythrocyte sedimentation rate, exhibited a distinct pattern, with a significant decrease in the number of tests in 2020, followed by a recovery to near predicted values in 2021. It is important to note that in the context of the Korean health insurance payment system, these tests are relatively freely prescribed, which may have influenced their usage. In contrast, the number of procalcitonin tests, which are typically reserved for cases of suspected Systemic Inflammatory Response Syndrome or sepsis, did not see such a pronounced drop. This differential usage may be attributed to the more restrictive guidelines surrounding the prescription of procalcitonin tests. However, no significant changes were observed in tests for vector-borne infections, such as Leptospira, scrub typhus, and hantavirus, as well as chronic viral infections including viral hepatitis, human cytomegalovirus, and Epstein-Barr virus. Likewise, tests for nosocomial infections, such as Clostridioides difficile, and Helicobacter pylori following gastrointestinal endoscopy, showed no significant variations. Notably, the frequency of human immunodeficiency virus testing experienced a significant decrease after January 2020, and this lower rate was consistently maintained for two years.
We investigated changes in clinical microbiological testing trends in South Korea during the COVID-19 pandemic. Our study stands out because unlike most similar studies that relied on national reporting of notifiable diseases, our research utilized data on the number of prescribed diagnostic tests, which provided a comprehensive understanding of nationwide changes in infectious diseases. This approach can serve as a model for other countries.
Our findings align with various domestic and international studies that have confirmed similar trends. In particular, the Korean studies revealed a decrease in notifiable infectious diseases and a decrease in the detection rate of respiratory viruses after the COVID-19 period.791011 Reports echoing similar trends have been observed globally.12 Our study adds to the accumulating evidence that while the application of NPIs can potentially lower the incidence of various infectious diseases, their effects might not be solely attributable to these measures. It is important to consider that alongside NPIs, other factors such as decreased overall healthcare utilization, a generalized reduction in infectious diseases, and incidental measures like temperature checks could collectively contribute to the observed decrease in diagnostic suspicion towards infectious diseases. These considerations should play an integral role in shaping public health policies and strategies for managing infectious disease outbreaks.
Nevertheless, it is apparent from our study that the comprehensive changes following the COVID-19 pandemic, rather than NPIs alone, do not seem to markedly influence nosocomial infections, chronic viral infections, or vector-based infections. Indeed, the observed changes in inflammatory markers suggest a significant reduction in overall infections. However, given the high proportion of respiratory viral infections in the overall disease burden, the specific impacts of the multifaceted changes following COVID-19 on individual infectious diseases warrant careful consideration. Further research is necessary to tease out the factors contributing to the differential impacts of these widespread changes on various types of infectious diseases.
This study has several limitations. First, logistical factors may have influenced the results because this study was based on real-world data, not on data specifically collected for research purposes. For example, the decrease in the number of claims in February each year, as shown in Fig. 1, could be influenced by factors like the Korean New Year’s Day and student vacations during that time. Second, our study presents the results of the number of tests, not the confirmation of infectious disease; therefore, the results are largely dependent on medical utilization. Additionally, the COVID-19 situation in Korea was relatively stable in 2020 and 2021, and the disruption to the healthcare system due to COVID-19 was relatively minimal. Future research could explore the effects of COVID-19 on the healthcare system and the incidence of infectious disease. Lastly, the analysis did not differentiate between sample types. Bacterial culture tests can be performed on blood, urine, the respiratory tract, and body fluids; however, the distinction is not made in the NHIS database.
In conclusion, our results may provide a broader perspective than those derived from traditional surveillance systems, enhancing our understanding of pandemics and improve infectious disease surveillance systems.
Notes
Funding: This study was supported by the National Research Foundation of Korea (NRF) grant funded by the Korean government (MSIT) (grant number: NRF-2021R1A5A2030333) and grant of the project for Infectious Disease Medical Safety, funded by the Ministry of Health & Welfare, Republic of Korea (grant number: HG22C0094). Also supported by a grant (22183MFDS431) from ministry of food and drug safety in 2022. The sponsor of the study was not involved in the study design, data analysis, data interpretation, writing of the report, or the decision to submit the study results for publication.
References
1. Downey LE, Gadsden T, Vilas VD, Peiris D, Jan S. The impact of COVID-19 on essential health service provision for endemic infectious diseases in the South-East Asia region: a systematic review. Lancet Reg Health Southeast Asia. 2022; 1:100011. PMID: 35769109.

2. Min KD, Kang H, Lee JY, Jeon S, Cho SI. Estimating the effectiveness of non-pharmaceutical interventions on COVID-19 control in Korea. J Korean Med Sci. 2020; 35(35):e321. PMID: 32893522.

3. Yang B, Huang AT, Garcia-Carreras B, Hart WE, Staid A, Hitchings MD, et al. Effect of specific non-pharmaceutical intervention policies on SARS-CoV-2 transmission in the counties of the United States. Nat Commun. 2021; 12(1):3560. PMID: 34117244.

4. Geng MJ, Zhang HY, Yu LJ, Lv CL, Wang T, Che TL, et al. Changes in notifiable infectious disease incidence in China during the COVID-19 pandemic. Nat Commun. 2021; 12(1):6923. PMID: 34836947.

5. Wang P, Xu Y, Su Z, Xie C. Impact of COVID-19 pandemic on influenza virus prevalence in children in Sichuan, China. J Med Virol. 2023; 95(1):e28204. PMID: 36217691.

6. Huh K, Kim YE, Ji W, Kim DW, Lee EJ, Kim JH, et al. Decrease in hospital admissions for respiratory diseases during the COVID-19 pandemic: a nationwide claims study. Thorax. 2021; 76(9):939–941. PMID: 33782081.

7. Huh K, Jung J, Hong J, Kim M, Ahn JG, Kim JH, et al. Impact of nonpharmaceutical interventions on the incidence of respiratory infections during the coronavirus disease 2019 (COVID-19) outbreak in Korea: a nationwide surveillance study. Clin Infect Dis. 2021; 72(7):e184–e191. PMID: 33150393.

8. Yun HE, Ryu BY, Choe YJ. Impact of social distancing on incidence of vaccine-preventable diseases, South Korea. J Med Virol. 2021; 93(3):1814–1816. PMID: 33079384.

9. Kim DH, Nguyen TM, Kim JH. Infectious respiratory diseases decreased during the COVID-19 pandemic in South Korea. Int J Environ Res Public Health. 2021; 18(11):6008. PMID: 34205018.

10. Shi HJ, Kim NY, Eom SA, Kim-Jeon MD, Oh SS, Moon BS, et al. Effects of non-pharmacological interventions on respiratory viruses other than SARS-CoV-2: analysis of laboratory surveillance and literature review from 2018 to 2021. J Korean Med Sci. 2022; 37(21):e172. PMID: 35638198.

11. Kang JM, Kim YE, Huh K, Hong J, Kim DW, Kim MY, et al. Reduction in Kawasaki disease after nonpharmaceutical interventions in the COVID-19 era: a nationwide observational study in Korea. Circulation. 2021; 143(25):2508–2510. PMID: 34092115.

12. Wang X, Xu H, Chu P, Zeng Y, Tian J, Song F, et al. Effects of COVID-19-targeted nonpharmaceutical interventions on children’s respiratory admissions in China: a national multicenter time series study. Int J Infect Dis. 2022; 124:174–180. PMID: 36241166.

SUPPLEMENTARY MATERIALS
Supplementary Table 1
Comparison of the average monthly number of clinical microbiology tests performed before and after COVID-19
Supplementary Fig. 1
Monthly changes in the number of tests before and after COVID-19, with a two-year forecast presented without COVID-19.
Fig. 1
Monthly changes in the number of tests before and after COVID-19, with a two-year forecast presented without COVID-19. (A) IgM, Mycoplasma; (B) Urine antigen, Legionella; (C) PCR, Tuberculosis; (D) IGRA for Tuberculosis; (E) ESR; (F) CRP; (G) ASO, Quantitative; (H) Procalcitonin; (I) Culture and AST; (J) Culture and AST including anaerobes; (K) Gram stain; (L) Antibody, Leptospira; (M) Antibody, Scrub typhus; (N) Antibody, Hantavirus; (O) HBsAg; (P) Antibody, HCV; (Q) Antibody, HIV; (R) PCR, CMV; (S) PCR, EBV; (T) Toxin, Clostridioides difficile; (U) Urease test for Helicobacter pylori.
COVID-19 = coronavirus disease 2019, PCR = polymerase chain reaction, IGRA = interferon gamma release assay, ESR = erythrocyte sedimentation rate, CRP = C-reactive protein, ASO = anti-streptolysin O, AST = antimicrobial susceptibility test, HBsAg = hepatitis B virus surface antigen, HCV = hepatitis C virus, HIV = human immunodeficiency virus, CMV = human cytomegalovirus, EBV = Epstein-Barr virus.
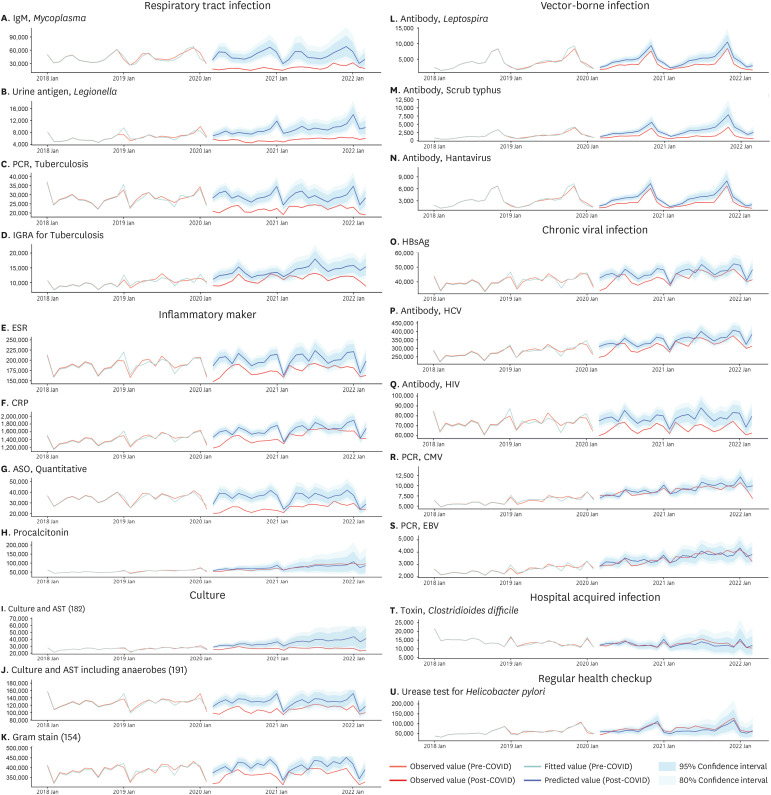
Table 1
Comparison of the average monthly number of clinical microbiology tests performed before and after COVID-19
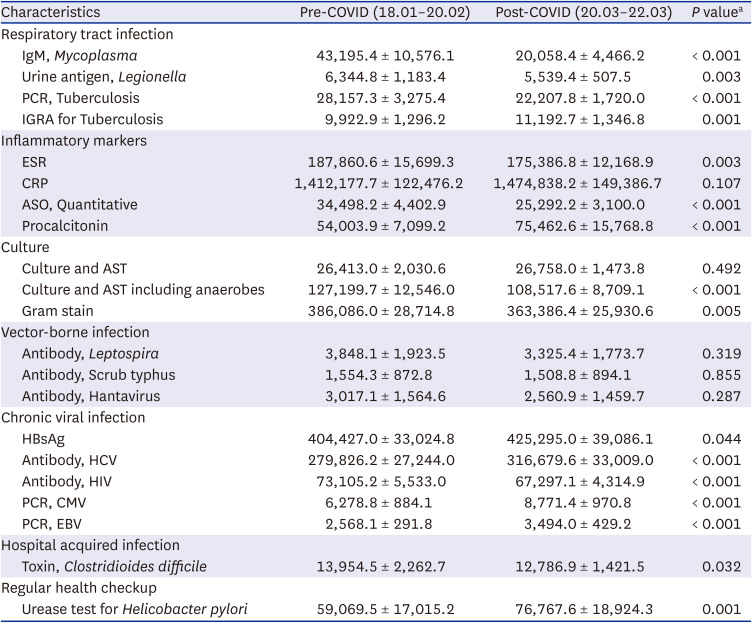
COVID-19 = coronavirus disease 2019, PCR = polymerase chain reaction, IGRA = interferon gamma release assay, ESR = erythrocyte sedimentation rate, CRP = C-reactive protein, ASO = anti-streptolysin O, AST = antimicrobial susceptibility test, HBsAg = hepatitis B virus surface antigen, HCV = hepatitis C virus, HIV = human immunodeficiency virus, CMV = human cytomegalovirus, EBV = Epstein-Barr virus.
aComparisons made via independent t-test.




 PDF
PDF Citation
Citation Print
Print



 XML Download
XML Download