Abstract
Background
Brain metastases of peri-Rolandic area is crucial as it directly impacts the quality of life for cancer patients. Surgery or stereotactic radiosurgery (SRS) is considered for peri-Rolandic brain metastases as for other brain metastases. However, the benefit of each treatment modality on functional outcome has not been clearly defined for this tumor. The purpose of this study is to compare the functional course of each treatment and to suggest an effective treatment for patients’ quality of life.
Methods
Fifty-two patients who had undergone SRS or surgery for brain metastasis confirmed by enhanced MRI were enrolled retrospectively. Overall survival (OS), progression free survival (PFS), and functional outcomes were estimated using the Kaplan-Meier method, univariate, multivariate analysis, and Cox proportional hazards regression.
Results
Median OS and PFS were 13.3 months and 8.9 months in our study population. Treatment modalities were not significant factors for OS and PFS. Extracranial systemic cancer progression was significant factor for both parameters (p=0.030 for OS and p=0.040 for PFS). Median symptom improvement (improvement of at least 1 grade after surgery compared to preoperative state) time was significantly shorter in surgery group than in the SRS group (10.5 days vs. 37.5 days, p=0.034).
Brain metastases (BMs) are a significant cause of morbidity and mortality among cancer patients [1]. The incidence of BMs ranges from 8.2 per 100,000 person-years in the general population to 9% to 17% among cancer patients, as reported in various studies [23]. Despite advancements in neurosurgical, radiation, and systemic chemotherapy treatments, the median survival time remains at 13 months [123]. The peri-Rolandic area is particularly crucial as it directly impacts the quality of life for cancer patients. Metastases in this region can result in motor weakness, leading to a decline in performance status and potentially limiting treatment options [4]. Intravenous corticosteroid injections can provide temporary improvement in motor symptoms. However, prolonged corticosteroid use may lead to complications such as hyperglycemia, iatrogenic adrenal insufficiency, or Cushing’s syndrome [56]. The optimal treatment for metastases in eloquent areas is still a matter of debate [67]. Concerns regarding motor weakness after surgical treatment for tumors in the Rolandic area have diminished due to recent advancements in intraoperative neuro-monitoring systems and diffuse tensor MRI mapping systems [4]. Delayed chemotherapy following surgical resection poses concerns related to wound complications or the patient’s general condition. Stereotactic radiosurgery (SRS) serves as a useful alternative option for deeper areas, elderly patients, or multiple lesions. However, it also carries a risk of radiation toxicities such as radio necrosis [8910]. This study aims to compare the clinical outcomes of peri-Rolandic BMs between surgical resection for primary treatment and SRS only for life, further evaluating the efficacy of each treatment in improving the patient’s quality of life.
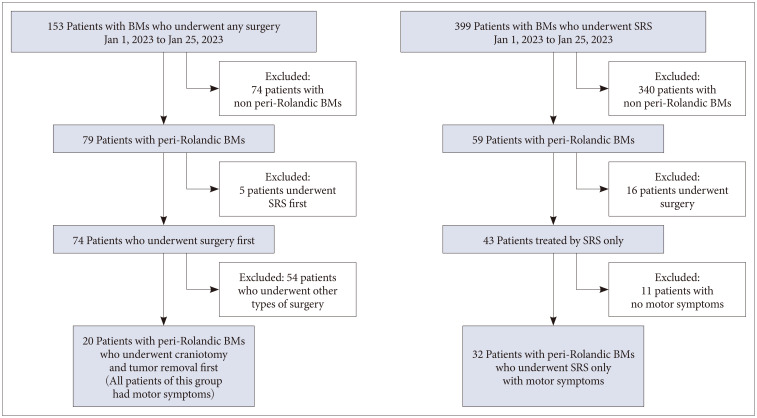
This retrospective study was conducted at Gachon University Gil Medical Center following IRB approval (GDIRB2023-208). Because of its retrospective manner, informed consent was waived. This study included patients who were treated for metastatic brain tumors from January 1, 2013, to January 30, 2023. The inclusion criteria consisted of patients aged 18 years or older with confirmed metastasis in brain imaging who underwent tumor removal with craniotomy or SRS (Novalis Tx, Elsan, Paris, France). Only patients who had surgery for their first treatment were included in surgery group. In surgery group, exclusion criteria were made for patients who underwent Ommaya reservoir insertion, extra-ventricular drainage, ventriculoperitoneal shunt, or stereotactic biopsy. Among the patient group, those with motor symptoms were selected based on documented symptoms in medical records and pre-treatment MRI. The Rolandic area, defined as the region anterior to the central sulcus, was identified using specific signs (L sign, Bracket sign, and U sign) in the MRI images [1112]. To account for tumor-induced destruction of normal structures, multiple methods were simultaneously employed to define the area. Only patients who were treated only with SRS were included in SRS group. All of patients in surgery group had motor symptoms. In this study, clinical outcomes were evaluated only for patients with peri-Rolandic area tumor, and a total of 52 patients were enrolled in the study, including 20 patients in the surgery group and 32 patients in the SRS group.
Regarding the primary cancer workup, all patients underwent chest and abdomen CT scans. Whenever possible, positron emission tomography CT scans were also conducted, along with histopathological confirmation obtained through procedures such as needle aspiration or post-surgical pathology, ensuring the inclusion of patients with a confirmed diagnosis of primary cancer. In cases involving multiple surgeries and SRS, the analysis focused on the initial treatment administered. Specifically, within the SRS group, patients who received adjuvant SRS were included in the surgical patient cohort to facilitate further examination. All MRI scans were conducted using a 3.0-T MRI scanner (MAGNETOM Skyra, Vida 3T; Simens Healthineers, Erlangen, Germany). For the SRS group, patients were examined 1 month after radiosurgery and then every 3 months. MRI and enhancement CT scans were alternately conducted every 3 months for the first year, and then every 4–6 months indefinitely. For the surgery group, the extent of resection was evaluated with postoperative brain MRI within 48 hours after surgery. The patients were discharged on the 7th day post-surgery. After discharge, radiographic evaluation was performed similar to the radiosurgery group.
The treatment modality was determined by the general consensus, the experience of physicians, and the patient’s condition. Guidelines such as American Society of Radiation Oncology (ASTRO) were often considered [13]. In general, surgery was preferred for the tumor of 3–4 cm or more in size with easy to access surgically. Patients with poor general condition, multiple lesions, and deep location tend to be treated by SRS.
Craniotomy was performed using neuro-navigation system (Curve Navigation, Brainlab, Munich, Germany) with a standard stereotactic guide and intraoperative neuromonitoring. Diffusion tensor imaging MRI was used to evaluate the deviation of motor fibers and minimize damage to the motor cortex.
For SRS, a gadolinium-enhanced T1-weighted MRI with a 1-mm slice thickness was used. Patients underwent CT imaging with individualized localizing stereotactic head frames, using a 2-mm slice thickness CT scan for simulation on the day of surgery or the day before. The CT scans were fused with MRI using Brainlab treatment planning software (iPlan RT image 4.1.2) to delineate the gross tumor volume (GTV). For patients who did not undergo open surgery, the GTV was defined as the contrast-enhanced tumor and adjacent abutting meninges. For patients who underwent open surgery, the GTV was defined as the surgical cavity, residual enhancing tumor, and adjacent abutting meninges. The SRS plan consisted of an 80% isodose line with a median dose of 21 Gy (range: 15–23 Gy) delivered in a single fraction or each 11 Gy (range: 9–12 Gy) delivered in three fractions. The SRS plan determined by size and number of tumors. Guidelines such as ASTRO were often considered. For patients with BMs measuring <2 cm diameter, single fraction SRS with a dose of 20–24 Gy is recommended. For patients with BMs measuring ≥2 cm to <3 cm in diameter, single fraction SRS using 18 Gy or each 9–10 Gy delivered in three fractions is recommended. Tumors of other sizes were treated similarly to the previous mentions, referring to the guidelines [13].
Corticosteroid usage varied depending on the discretion of the treating physician, with the primary goal of symptom improvement. The dosage varied depending on the physician’s prescription, typically ranging from 8 mg to 20 mg per day during the acute phase. The dosage was gradually tapered based on symptom improvement, and in cases where symptoms recurred during steroid tapering, a low dose was continued. Steroids were primarily administered as a bolus when symptoms recurred.
The primary outcomes assessed were overall survival (OS) and progression-free survival (PFS), while the secondary outcome was the observed changes in motor symptoms. The analysis of symptoms was divided into two categories: functional outcomes documented in the medical records and the use of dexamethasone, including doses and duration of use. Relevant variables investigated in the analysis included sex, age, comorbidities (such as diabetes mellitus and hypertension), initial Karnofsky Performance Status (KPS), tumor size, laterality of symptoms, peri-tumoral edema, presence of multiple lesions, presence of cystic lesions, final KPS, administration of whole-brain radiation therapy, adjuvant chemotherapy, occurrence of systemic progression, and dexamethasone tapering schedule. For OS, patients who died were assigned the date of death based on insurance records, while surviving patients were censored at their last outpatient visit or the date of the last image workup. For PFS, the time of tumor progression was determined based on the image workup. Functional outcomes were assessed based on improvement or worsening of symptoms documented in the medical records. Improvement or worsening of symptoms were defined as a change in motor grade. Improvement was defined as follows: When the preoperative motor grade was less than 3, it refers to recovery to 3 or higher after surgery. When the preoperative motor grade was 3 or higher, it signifies an improvement of at least 1 grade after surgery. Worsening was defined as decreased motor grade of at least 1 grade after surgery.
IBM SPSS Statistics 27 (IBM Corp., Armonk, NY, USA) was utilized for the statistical analysis. The log-rank test was employed for categorical variables, while Cox regression analysis was utilized for both univariate and multivariate analysis. The independent factors and parameters included in the analysis were age (<65 vs. ≥65 years), pre-treatment KPS score (<70 vs. ≥70), number of BMs (1 vs. >1), tumor size (>2.5 cm vs. ≤2.5 cm), histology (lung vs. breast vs. colorectal vs. others), and extra-cranial disease (stable vs. active). Factors that exhibited both clinical importance and a p<0.05 were incorporated into the multivariate analysis. The primary outcomes assessed were OS and PFS, while the secondary outcome was symptom improvement. The significance among different patient groups was evaluated using the log-rank test, considering a p<0.05 as statistically significant.
The characteristics of the enrolled patient population are presented in Fig. 1 and Table 1. The median age was 64 (range: 42–85), and the median tumor size was 2.7 cm (range: 1.2–7.2 cm). Single metastasis accounted for 63.5% of cases. The median KPS score at the time of surgery was 70, and 21.2% of patients had a performance state with a KPS score below 70. The most common primary cancer was lung cancer, representing 57.7% of cases. Table 2 describes the characteristics of patients for each treatment modality. The individual characteristics of the patients who underwent surgical treatment and those who received SRS alone were similar, as shown in Table 2. Tumor size was significantly larger in the surgery group (4.1 cm vs. 2.2 cm, p=0.001). Single metastasis was more frequent in the surgery group (80% vs. 53%, p=0.050). The median OS and PFS were longer in the surgical group, but the differences were not statistically significant (p=0.103 and p=0.298, respectively). The median initial KPS scores in both groups were not significantly different, with 70 (range: 40–80) in the surgical group and 70 (range: 40–90) in the SRS group (p=0.093). The proportion of patients with symptom improvement was higher in the surgical group, and the median time to symptom improvement was shorter in the surgical group compared to the SRS group (10.5 days vs. 37.5 days, p=0.034). The use and dosage of dexamethasone as an indicator of symptom change showed no significant differences between the two groups. First, the median duration of steroid treatment was evaluated, which was 8.5 days and 7.5 days in the surgical and SRS groups, respectively (p=0.938). The second was the proportion of patients who restarted steroids, which was 45% and 40.6% in the surgical and SRS groups, respectively (p=0.756).
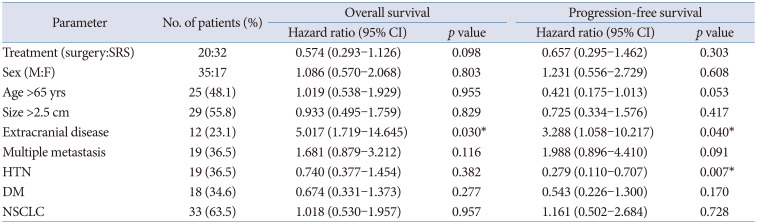
In the total cohort, systemic disease progression was found to significantly affect OS (HR 5.017, 95% confidence interval [CI] 1.719–14.645). The surgical group also demonstrated a significant association with this factor (HR 4.691, 95% CI 1.006–21.877). However, in the SRS group, this factor did not reach significance. Instead, tumor size showed a significant association (HR 2.155, 95% CI 1.411–3.292). The treatment method (surgery or SRS) was not found to be a significant factor in OS (Table 3). Kaplan-Meier survival analysis was performed, revealing median survival times of 13.3 months for the total cohort, 17.9 months for the surgical group, and 10.4 months for the SRS-alone group. There was no significant difference in median survival between the surgical and SRS groups (Fig. 2). Multivariate analysis did not identify any significant factors among the variables considered.
Similar trends were observed in PFS. The factor influencing PFS in the total cohort was systemic disease progression, similar to OS (HR 3.288, 95% CI 1.058–10.217). Additionally, hypertension showed a favorable condition (p=0.007). In subgroup analysis, the PFS of the surgical group exhibited systemic disease progression as significant factor (HR 9.031, 95% confidence interval 1.052–77.498), while multiple BMs showed significance (HR 0.026, 95% CI 1.226–25.534). However, no significant factors were found for patients who received SRS alone. Similar to OS, the treatment methods (surgery or SRS) were not a significant factor in PFS (Table 3). The median PFS for the total cohort was 8.9 months, 13.0 months for the surgical group, and 6.4 months for the SRS-alone group. There was no significant difference in median PFS between the surgical and SRS groups (Fig. 2). Multivariate analysis did not identify any significant factors among the variables considered, both in PFS and OS.
This study focused on the analysis of patient symptoms, particularly in relation to the management of symptoms using dexamethasone. Changes in patient symptoms and dexamethasone dosage were analyzed.
Firstly, in the total cohort, the median time to symptom improvement was 10.5 days for the surgery group and 37.5 days for the SRS group (p=0.034) (Table 2). Among patients under the age of 65, there was a 2.1-fold improvement in symptoms compared to the control group (odds ratio [OR] 2.1, 95% CI 1.052–4.367). Patients with primary cancer of non-small cell lung cancer (NSCLC) demonstrated better functional outcomes compared to those with other primary cancers (OR 2.753, 95% CI 1.232–6.152).
Among the subgroups, the surgical group showed that hypertension improved symptoms (OR 5.301, 95% CI 1.504–18.679), while the SRS group exhibited a similar trend to the total cohort, with being under the age of 65 associated with improvement in symptoms (OR 3.584, 95% CI 1.353–9.524). NSCLC also demonstrated improved symptoms compared to other primary cancers (OR of 3.629, 95% CI 1.052–12.518).
Regarding steroid-related factors, there was no significant correlation between steroid tapering and the treatment methods (surgery or SRS). Similarly, in the total cohort, there were no significant factors associated with steroid tapering or restart. However, in the surgical group, the likelihood of restarting steroids was higher for female patients (OR 6.540, 95% CI 1.601–26.714).
Complications after SRS were present as shown in the Fig. 3. Acute symptoms mainly occurred within 7 weeks after SRS and included minor symptoms such as dizziness, headache, and nausea. Subsequently, radiation-related necrosis was observed on imaging after several months, accompanied by seizures, sensory changes, motor weakness, and others. A total of 31% of patients experienced complications. There was no significant complication in surgery group.
Tumors located in the Rolandic area pose significant challenges for neurosurgeons, regardless of their type. Clinical outcomes of tumors in this location have been reported for various tumor groups [14], including meningioma meningioma [15] and glioma [4]. Traditionally, surgical intervention for brain tumors in this area was avoided. However, recent research has shown that the use of advanced intraoperative neuro-monitoring, diffusion tensor MRI, and mapping techniques can help minimize motor symptoms after surgery [15].
The epidemiology observed in this study did not differ significantly from previous studies [16]. In the surgical group, tumors tended to be larger in size, and single metastasis was more common. These findings align with the recommended guidelines and practices [13].
In previous studies analyzing patients with BMs, no significant differences in median OS and PFS were observed based on treatment methods, but systemic progression was identified as a contributing factor [91017]. Similarly, for metastasis in the peri-Rolandic area, there were no significant differences in OS and PFS among different treatment methods. The most significant factor influencing outcomes was the progression of extra-cranial lesions. When analyzing each patient group separately, both groups showed systemic progression as a significant factor. In the SRS group, tumor size was additionally found to be significant (HR 2.155, 95% CI 1.411–3.292). Although previous studies have reported that KPS influences OS, it did not have a significant impact in this study [1917].
The decline in motor function is believed to be associated with a deterioration in KPS, which may impact the administration of systemic chemotherapy or aggressive treatments [1]. However, similar to the previous discussions, no significant differences were observed in this regard. Previous studies have also identified various factors, but the common factor was systemic control of primary cancer, which was similarly observed in this patient group [917].
Regarding PFS, both patient groups exhibited a 50% rate of local progression, and systemic progression of cancer remained a significant factor for PFS in the total cohort, consistent with the previous analysis [818]. Additionally, hypertension emerged as a factor that inhibits progression, and there have been studies suggesting that angiotensin receptor blockers, commonly used for hypertension, can reduce brain damage. Further literature review is needed to explore this topic [19]. In subgroup analysis, multiple metastases were identified as a risk factor in the surgical group, while no factors associated with progression were found in the SRS group.
In previous studies, patients with BMs exhibited different survival rates depending on the primary cancer [1]. However, no significant results were found regarding OS or PFS based on tumor origin.
Among patients who underwent surgery and SRS, approximately 63% experienced functional improvement. When analyzed by subgroups, the improvement rate was approximately 70% in the surgical group and 59.4% in the SRS group, suggesting a slightly higher improvement in the surgical group, approximately 10% higher. The median time to symptom improvement was 10.5 days and 37.5 days, respectively (p=0.034). These findings indicate that surgical treatment resulted in faster symptom improvement. It suggests that surgical intervention can provide prompt symptom relief, potentially improving the patient’s overall quality of life by reducing the mass effect. Most of the patients exhibited neurological deficits in their limbs with a motor grade of 3 or higher before treatment. So, improvement mainly represent an improvement of at least 1 grade after treatment. Most of patient with less than motor grade of 3 before treatment experienced similar or deterioration of symptom after treatment.
Steroid tapering was successfully achieved within approximately 1 week in both subgroups, with around 90% of patients being able to discontinue steroid use. However, there was no significant association observed between treatment method and steroid tapering (p=0.805). In subgroup analysis, the surgical group required approximately 1 day longer for tapering (8.5 days), but this difference was not statistically significant. Steroids are medications that are not recommended for long-term use due to known complications. Therefore, even if symptoms persisted slightly, low-dose tapering or discontinuation of steroids was preferred. Throughout the treatment course, many patients reported complications such as nausea and hyperglycemia [7]. In the surgical group, approximately 85% of patients experienced symptom improvement within 10 days, allowing for a faster tapering of steroids. In contrast, in the SRS group, it took approximately 1–2 months for symptom improvement in 42% of cases, and steroid tapering was performed if there was no worsening of symptoms.
Regarding symptom outcomes, in the total cohort, being over 65 years old was identified as a significant adverse risk factor (p=0.036). While previous studies mostly found that age did not significantly impact outcomes, there are cases, such as in this study, where older age can influence outcomes [9]. Interestingly, in the case of NSCLC, symptoms exhibited a better prognosis, which may be attributed to the advancements in chemotherapy and the improved efficacy of NSCLC-targeting drugs. This finding is in contrast to previous studies that reported an adverse effect of NSCLC on survival rates [1]. In the subgroup analysis, hypertension was identified as a favorable factor for symptom improvement in the surgical group, which aligns with the previously discussed context. The SRS group demonstrated similar results to the total cohort.
Various treatments, including systemic chemotherapy and tyrosine kinase inhibitors, are being explored for the management of BMs. While previous drugs had limited ability to cross the blood-brain barrier, there are now many drugs with improved permeability. In the future, not only surgery and SRS but also systemic chemotherapy will likely be widely employed for BMs. Further research is needed, particularly for lesions located in the motor area, as they play a critical role in determining patients’ quality of life.
When symptoms manifest, factors such as tumor size, edema size, and the presence of multiple lesions can potentially impact treatment outcomes. However, in most cases, these factors did not have a significant influence on outcomes.
Among the surgical patient group, there were individuals who also underwent SRS, and their complications were investigated alongside the SRS-only group. In this study, the focus was mainly on investigating the patients’ symptoms, as indicated by the corresponding ratios mentioned in Fig. 3. The main complications observed were neurologic deterioration, headache, and seizures, which align with findings from previous studies [132021]. Generally, infection, wound dehiscence, postoperative hematoma, and seizure could be major concerns after surgery. But there were no significant complications specifically associated with surgery, likely due to the small size of the patient group. Major concern is neurologic deterioration after surgery in eloquent area. With recent techniques like intraoperative navigation system and intraoperative neuromonitoring systems, and favorable result in this study, that concerns could be reduced.
Since this is a retrospective study, the conditions among patient groups were not consistent. There are differing recommendations for surgery and SRS based on the general consensus, and this trend was also reflected in the characteristics of the patients in our study. For example, the group that underwent surgery often had larger tumor sizes, while the group that underwent SRS tended to have smaller tumors but more frequently had multiple lesions and poorer overall health conditions. These additional factors may have influenced the results. Therefore, it may be necessary to conduct future randomized controlled trials under similar conditions.
Surgery and SRS for the peri-Rolandic BMs did not have a significant impact on OS or PFS. In terms of symptom improvement, the surgically treated patient group demonstrated a significantly faster rate, which can contribute to a positive quality of life for the remaining duration of the patient’s life.
Notes
Author Contributions:
Conceptualization: Dong-Won Shin.
Data curation: Jun Hyeok Jung.
Formal analysis: Jun Hyeok Jung.
Methodology: Kawngwoo Park.
Project administration: Dong-Won Shin.
Supervision: Gi-Take Yee.
Validation: Woo-Kyung Kim.
Visualization: Eun Young Kim.
Writing—original draft: Jun Hyeok Jung.
Writing—review & editing: Chan-Jong Yoo.
Availability of Data and Material
The datasets generated or analyzed during the study are available from the corresponding author on reasonable request.
References
1. Sacks P, Rahman M. Epidemiology of brain metastases. Neurosurg Clin N Am. 2020; 31:481–488. PMID: 32921345.

2. Kim T, Song C, Han JH, Kim IA, Kim YJ, Kim SH, et al. Epidemiology of intracranial metastases in Korea: a national cohort investigation. Cancer Res Treat. 2018; 50:164–174. PMID: 28324921.

3. Cagney DN, Martin AM, Catalano PJ, Redig AJ, Lin NU, Lee EQ, et al. Incidence and prognosis of patients with brain metastases at diagnosis of systemic malignancy: a population-based study. Neuro Oncol. 2017; 19:1511–1521. PMID: 28444227.

4. Magill ST, Han SJ, Li J, Berger MS. Resection of primary motor cortex tumors: feasibility and surgical outcomes. J Neurosurg. 2018; 129:961–972. PMID: 29219753.

5. Hempen C, Weiss E, Hess CF. Dexamethasone treatment in patients with brain metastases and primary brain tumors: do the benefits outweigh the side-effects? Support Care Cancer. 2002; 10:322–328. PMID: 12029432.

6. Soffietti R, Cornu P, Delattre JY, Grant R, Graus F, Grisold W, et al. EFNS guidelines on diagnosis and treatment of brain metastases: report of an EFNS Task Force. Eur J Neurol. 2006; 13:674–681. PMID: 16834697.

7. Ryken TC, McDermott M, Robinson PD, Ammirati M, Andrews DW, Asher AL, et al. The role of steroids in the management of brain metastases: a systematic review and evidence-based clinical practice guideline. J Neurooncol. 2010; 96:103–114. PMID: 19957014.

8. Redmond KJ, De Salles AAF, Fariselli L, Levivier M, Ma L, Paddick I, et al. Stereotactic radiosurgery for postoperative metastatic surgical cavities: a critical review and International Stereotactic Radiosurgery Society (ISRS) practice guidelines. Int J Radiat Oncol Biol Phys. 2021; 111:68–80. PMID: 33891979.

9. Fuentes R, Osorio D, Expósito Hernandez J, Simancas-Racines D, Martinez-Zapata MJ, Bonfill Cosp X. Surgery versus stereotactic radiotherapy for people with single or solitary brain metastasis. Cochrane Database Syst Rev. 2018; 8:CD012086. PMID: 30125049.

10. González L, Castro S, Villa E, Zomosa G. Surgical resection versus stereotactic radiosurgery on local recurrence and survival for patients with a single brain metastasis: a systematic review and meta-analysis. Br J Neurosurg. 2021; 35:703–713. PMID: 34431733.

11. Naidich TP, Blum JT, Firestone MI. The parasagittal line: an anatomic landmark for axial imaging. AJNR Am J Neuroradiol. 2001; 22:885–895. PMID: 11337334.
12. Wagner M, Jurcoane A, Hattingen E. The U sign: tenth landmark to the central region on brain surface reformatted MR imaging. AJNR Am J Neuroradiol. 2013; 34:323–326. PMID: 22821920.

13. Schiff D, Messersmith H, Brastianos PK, Brown PD, Burri S, Dunn IF, et al. Radiation therapy for brain metastases: ASCO guideline endorsement of ASTRO guideline. J Clin Oncol. 2022; 40:2271–2276. PMID: 35561283.

14. Yaeger KA, Nair MN. Surgery for brain metastases. Surg Neurol Int. 2013; 4(Suppl 4):S203–S208. PMID: 23717791.

15. Raffa G, Picht T, Scibilia A, Rösler J, Rein J, Conti A, et al. Surgical treatment of meningiomas located in the rolandic area: the role of navigated transcranial magnetic stimulation for preoperative planning, surgical strategy, and prediction of arachnoidal cleavage and motor outcome. J Neurosurg. 2019; 133:107–118.

16. Roos DE, Smith JG, Stephens SW. Radiosurgery versus surgery, both with adjuvant whole brain radiotherapy, for solitary brain metastases: a randomised controlled trial. Clin Oncol (R Coll Radiol). 2011; 23:646–651. PMID: 21592754.

17. Krist DT, Naik A, Thompson CM, Kwok SS, Janbahan M, Olivero WC, et al. Management of brain metastasis. Surgical resection versus stereotactic radiotherapy: a meta-analysis. Neurooncol Adv. 2022; 4:vdac033. PMID: 35386568.

18. Sahgal A, Ruschin M, Ma L, Verbakel W, Larson D, Brown PD. Stereotactic radiosurgery alone for multiple brain metastases? A review of clinical and technical issues. Neuro Oncol. 2017; 19(suppl 2):ii2–ii15. PMID: 28380635.

19. Vadhan JD, Speth RC. The role of the brain renin-angiotensin system (RAS) in mild traumatic brain injury (TBI). Pharmacol Ther. 2021; 218:107684. PMID: 32956721.

20. Eaton BR, LaRiviere MJ, Kim S, Prabhu RS, Patel K, Kandula S, et al. Hypofractionated radiosurgery has a better safety profile than single fraction radiosurgery for large resected brain metastases. J Neurooncol. 2015; 123:103–111. PMID: 25862006.

21. Minniti G, Clarke E, Lanzetta G, Osti MF, Trasimeni G, Bozzao A, et al. Stereotactic radiosurgery for brain metastases: analysis of outcome and risk of brain radionecrosis. Radiat Oncol. 2011; 6:48. PMID: 21575163.

Fig. 2
Kaplan-Meier’s survival curve for overall survival (A) and progression-free survival (B) of each group. SRS, stereotactic radiosurgery.
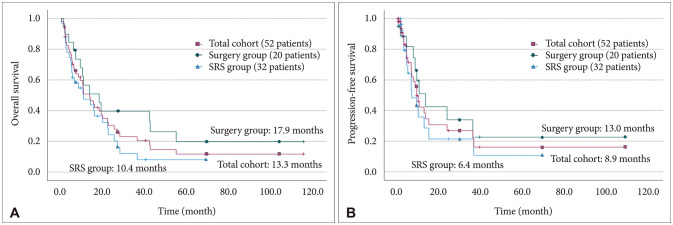
Fig. 3
Complications after radiosurgery. ETC, poor oral intakes, intractable hiccups, tremor, stomatitis, lymphedema.
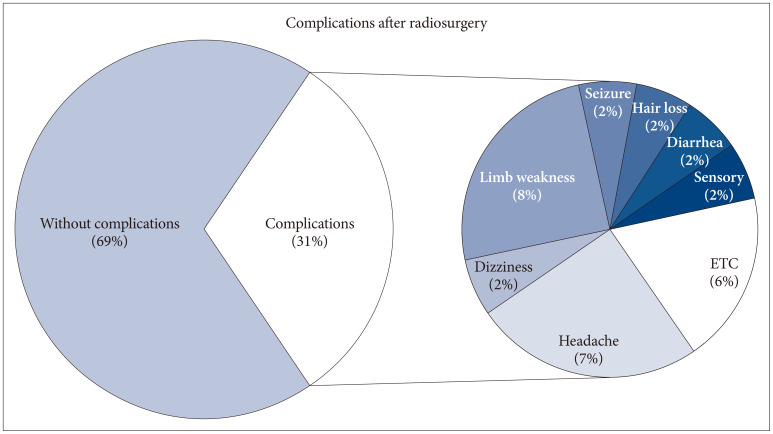
Table 1
Patient characteristics in the total study cohort
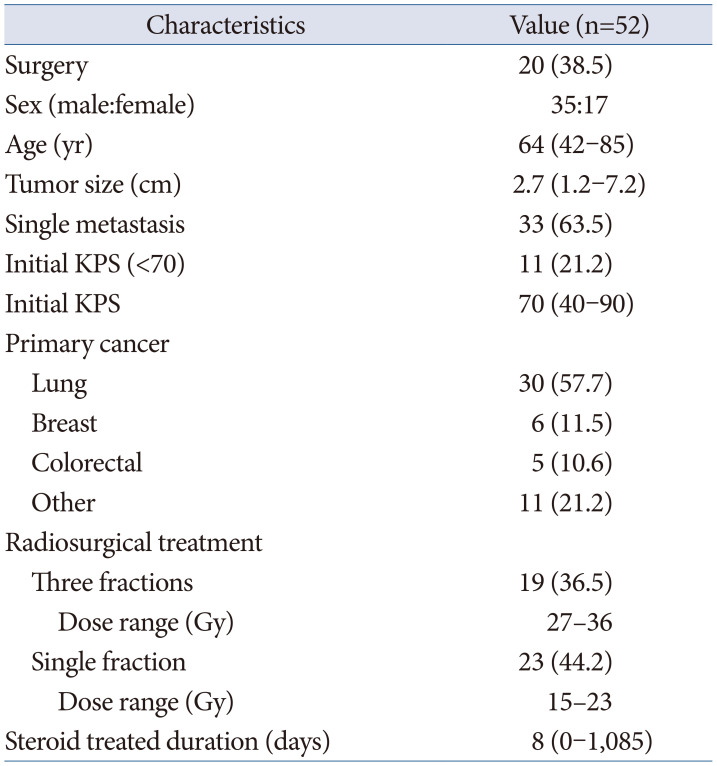
Table 2
Patient characteristics of each group
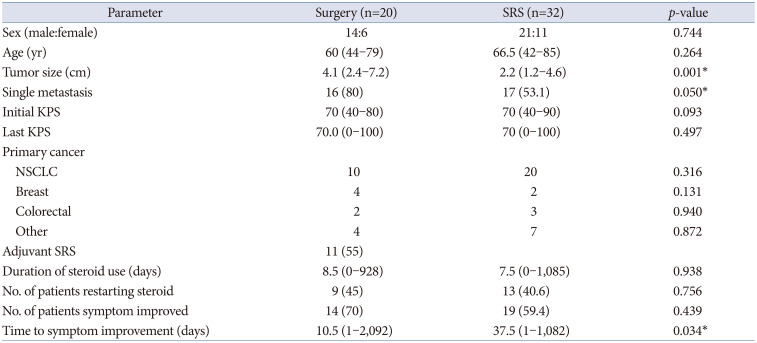
Table 3
Univariate analysis of overall survival, progression free survival




 PDF
PDF Citation
Citation Print
Print



 XML Download
XML Download