Abstract
Objective
The optic nerve sheath diameter (ONSD)/eyeball transverse diameter (ETD) ratio is a more reliable marker of intracranial pressure than the ONSD alone. We aimed to investigate the predictive value of the ONSD/ETD ratio (OER) for neurological outcomes in patients with aneurysmal subarachnoid hemorrhage (aSAH).
Methods
Adult patients with aSAH who visited the emergency department of a tertiary hospital connected to a South Korean university between January 2015 and December 2021 were included. Data on patient characteristics and brain computed tomography scan findings, including the ONSD and ETD, were collected using a predefined protocol. According to the neurological outcome at hospital discharge, the patients were divided into the unfavorable neurological outcome (UNO; cerebral performance category [CPC] score 3–5) and the favorable neurological outcome (FNO; CPC score 1–2) groups. The primary outcome was the association between the OER and neurological outcomes in patients with aSAH.
Results
A total of 171 patients were included in the study, of whom 118 patients (69%) had UNO. Neither the ONSD (p=0.075) nor ETD (p=0.403) showed significant differences between the two groups. However, the OER was significantly higher in the UNO group in the univariate analysis (p=0.045). The area under the receiver operating characteristic curve of the OER for predicting UNO was 0.603 (p=0.031). There was no independent relationship between the OER and UNO in the multivariate logistic regression analysis (adjusted odds ratio, 0.010; p=0.576).
Subarachnoid hemorrhage (SAH) is an emergent cerebrovascular disease and a devastating condition with high morbidity and mortality rates [6]. Early diagnosis and rapid treatment are important for improving the prognosis of patients with SAH. Early prediction of the prognosis of patients with SAH is also important when focusing on the limited medical resources available. The level of consciousness upon hospital admission is the most valuable early predictor of patient outcomes [5,6].
The optic nerve sheath diameter (ONSD) can be estimated noninvasively using neuroimaging, and a positive correlation between increased intracerebral pressure (ICP) and the ONSD has been reported in previous studies [7,18]. Non-contrast brain computed tomography (CT) is performed when patients with suspected SAH are admitted to the emergency department (ED), and the ONSD can be easily measured using brain CT. Thus, previous neuroimaging studies have shown an association between the prognosis of patients with SAH and ONSD [5]. However, there are inconsistent results regarding the value of the ONSD in patient outcomes in previous studies [16,24].
A recent meta-analysis showed a limitation in using the ONSD alone in post-cardiac arrest patients [4]. The authors found that ONSD alone may be affected by patient characteristics and that using the ONSD/eyeball transverse diameter (ETD) ratio (OER) was more reliable. We hypothesized that the OER is more reliable than the ONSD for the prognosis of patients with SAH. Thus, we investigated the association between the prognosis of patients with aneurysmal SAH (aSAH) and OER using initial brain CT in the ED.
This study was approved by the Institutional Review Board of Hanyang University Guri Hospital (GURI 2022-11-018). The requirement for informed consent was waived owing to the retrospective nature of the study. This retrospective observational cohort study included adult patients with aSAH who presented to a single medical center between January 2015 and December 2021.
This study included adult patients with aSAH who visited the ED and underwent brain CT to rule out acute stroke. All patients underwent aneurysmal repair, including surgery, clipping, or endovascular coiling. The following were the exclusion criteria : 1) age <18 years; 2) incomplete medical records; 3) previous intracranial lesions, such as those due to cerebral hemorrhage, those due to infarction, or brain tumor; 4) prior basic cerebral performance category (CPC) score of >1; 5) aneurysm that was not detected on brain CT; 6) trauma; 7) ocular problems that could influence the ONSD or ETD; 8) transferred from or to other hospitals; and 9) hopeless or terminally ill patients.
Data on the following patient characteristics were collected from the electronic health records : age, sex, height, weight, comorbidities, initial Glasgow coma scale (GCS) score, grade on the Hunt and Hess scale, grade on the modified World Federation of Neurological Surgeons (WFNS) scale, and time interval from ED admission to brain CT imaging and aneurysmal repair. We also determined the Fisher scale score, location of the aneurysm and presence of hydrocephalus, intraventricular hemorrhage (IVH), and findings of scan performed after aneurysmal repair using brain CT.
The included patients were classified into two groups according to the neurological outcomes at hospital discharge, and the CPC score was used as a neurological outcome at hospital discharge. Favorable and unfavorable neurological outcomes (FNOs and UNOs, respectively) were defined as CPC scores of 1–2 and 3–5, respectively. The OER on brain CT was measured and analyzed using between-group analysis. The primary outcome was the relationship between the OER and neurological outcomes in patients with aSAH.
Brain CT scans were obtained parallel to the orbital floor from the base of the skull to the vertex using 4-mm non-contrast continuous slices, according to standard methods. The ONSD and ETD were defined as the distances between the outer margins of the thick sheath layers surrounding the optic nerve and globe, respectively. The ONSDs were measured 3 mm behind the globe on both sides, and an axial scan with the maximum ETD of each eye was chosen. The ONSDs and ETDs of both eyes were averaged to obtain the mean values, and the OER was calculated. All measurements, including neurological outcomes, were blinded to the patient’s clinical information.
The images were enlarged by 450% using the PACS tool, and the window width and window level were moved 440 and 45 times, respectively. The mean ONSDs and ETDs were obtained for the right and left eyes. In addition, the OER was calculated. SOMATOM Definition Edge, SOMATOM Definition DS, and SOMATOM Sensation 16 CT scanners were used (Siemens Healthcare, Erlangen, Germany). The following parameters were set : 120 kVp, 250–500 mAs, and slice thickness 4–4.5 mm. All CT scans were stored in the PACS in the Digital Imaging and Communications in Medicine format.
The sample size was estimated based on a pilot study of 30 patients. Patients with FNO and UNO had OERs of 0.202± 0.035 and 0.215±0.032, respectively. The required sample size was 166 individuals (a-error, 0.05; power, 0.8; effect size, 0.388), and 183 participants were required in consideration of a 10% drop rate.
Continuous and categorical variables were reported as numbers with percentages and as medians with interquartile ranges, respectively. Student’s t-test or Wilcoxon rank-sum test was used in analyzing continuous variables. The chi-square test or Fisher’s exact test was used to compare categorical variables between the two groups. Differences with a p-value of <0.05 were considered statistically significant. The odds ratio of each covariant for UNO was determined using multivariate analysis with logistic regression, adjusting for confounding variables that were significant in the univariate analysis. Variables with p<0.1 in the univariate analysis with the OER were included in the multivariate analysis. The Hosmer-Lemeshow test was used to validate the calibrations of the logistic model. The area under the receiver operating characteristic (ROC) curve (AUC) was used to evaluate the prognostic usefulness of OER for predicting neurological prognosis, with a sensitivity of >1 indicating specificity. G*Power (3.1.9.6; Heinrich Heine University, Düsseldorf, Germany) was used for the study size calculation, and SPSS software (version 25.0; IBM, Armonk, NY, USA) was used for other statistical analyses.
Among the 828 patients with SAH enrolled in this study, 657 patients were excluded. Finally, 171 patients were included in the FNO (n=118, 69.0%) and UNO (n=53, 31.0%) groups (Fig. 1). The baseline patient characteristics are summarized in Table 1. The mean age of the included patients was 54.1±12.1 years, and 34.9% were male. Patients in the UNO group were much older than those in the FNO group, and the UNO group demonstrated a significantly higher incidence of diabetes mellitus. The UNO group had a lower GCS score and poorer grades on the Hunt and Hess and modified WFNS scales than the FNO group. The UNO group had a poorer Fisher scale score than the FNO group, as well as an increased incidence of hydrocephalus on brain CT and cerebral edema after aneurysmal repair (Table 2). There was no statistically significant difference in the location of the aneurysm between the two groups.
There were no significant differences in the ONSDs between the UNO and FNO groups (p=0.075; Table 3). Moreover, there was no significant difference in the ETDs across the groups (p=0.403). However, the OER was significantly higher in the UNO group than in the FNO group (0.213 vs. 0.205, p=0.045). The multivariate logistic regression analysis for UNO with baseline variables and the OER are summarized in Table 3. Age, initial GCS score, and presence of IVH were independently associated with UNO. However, there was no independent association between the OER and UNO in the multivariate analysis (p=0.576). In the ROC curve for the ONSD alone and OER, the AUC for predicting UNO was 0.597 (95% confidence interval [CI], 0.509–0.685; p=0.042) and 0.603 (95% CI, 0.515–0.691; p=0.031), respectively (Fig. 2).
In this study, the OER was significantly associated with neurological outcomes in patients with aSAH. However, after adjusting for confounders, there was no independent association between the OER and neurological outcomes. In addition, the OER had poor prognostic accuracy for predicting UNO in patients with aSAH.
Compared with aSAH, non-aSAH typically has a much more benign course and better neurological outcomes [1,11]. To reduce heterogeneity in the study population, we tried to exclusively include participants with aSAH. Patients with poorgrade SAH have high mortality rates and unfavorable neurological outcomes [22]. The high mortality and morbidity rates following aSAH are thought to be associated with delayed cerebral ischemia (DCI) [17]. Early brain damage may contribute to the development of DCI and UNO following aSAH, and both associated with severe increased ICP [17]. ICP increases sharply when blood suddenly leaks into the subarachnoid space and causes decreased cerebral perfusion pressure and cerebral ischemia. Particularly, in patients with poor-grade SAH, increased ICP is common in the early hours after aSAH [10,12].
Despite conflicting results regarding the association between ICP and outcomes in patients with aSAH, early ICP monitoring and management in patients with poor-grade aSAH may reduce mortality and unfavorable outcomes [3]. Intraventricular catheters and parenchymal probes are the monitoring devices most frequently used [3]. However, these invasive tools are not easily assessed in the ED and risk causing complications, such as bleeding, infection, and mechanical failure [2]. Although a physical examination is rapid and noninvasive, it has limited value for monitoring increased ICP. Thus, ED clinicians require a rapid technique to detect increased ICP.
Brain CT is performed in the ED to rule out acute stroke in patients with neurological impairment. The ONSD can be easily determined using imaging studies, such as those using brain CT. The optic nerve is encased in the optic nerve sheath that connects directly to the subarachnoid space and is filled with cerebrospinal fluid (CSF). Expansion of the optic nerve sheath by increased CSF causes dilatation of the ONSD; thus, the ONSD reflects the increased ICP [8,20,21]. Previous studies have reported a positive association between increased ICP and the ONSD [7,18]. The ONSD measured using brain CT demonstrated a linear correlation with ICP acquired via invasive monitoring [19]. However, patient characteristics, such as age, sex, height, weight, and head circumference, could impact the ONSD. Kim et al. [14] showed a correlation between male sex, high body mass index (BMI), and ONSD dilatation in healthy Asian adults using ultrasonography (US).
The OER could be used to adjust for individual characteristics that might influence the ONSD. In the multivariate analysis, only the ETD was independently related to the ONSD. In contrast, the OER was unrelated to age, sex, or BMI [13]. A retrospective study showed that the OER was a more reliable predictor of comatose status in patients with supratentorial lesions than the ONSD [23]. Cho et al. [4] found that the OER was more reliable than the ONSD in predicting neurological outcomes in out-of-hospital cardiac arrest survivors.
The characteristics of the modality used for ONSD measurement are an important point of contention. A recent meta-analysis reported that ONSD assessment using optic US was more reliable than using brain CT [15]. Given that the optic nerve sheath is not observed parallel to the brain CT scan, it appears obliquely rather than horizontally in the image. An accurate image of the ONSD can be produced with ocular US as opposed to brain CT because the ONSD can be estimated with an image perpendicular to the optic sheath. Additionally, clinicians can perform bedside US in an emergency setting, and sonographic training may enable rapid skill acquisition [7,18]. ICP monitoring can be performed using portable US. A portable ultrasound machine can be easily and noninvasively used to monitor ICP in patients with aSAH.
This study had some limitations. First, it was based on a retrospective review of medical records, which may have insufficient statistical power. Despite our efforts to collect many feasible variables, there may be unknown confounding factors, such as the time interval from symptom onset to admission to the ED, that could affect neurological outcomes in patients. Thus, well-designed, large-scale prospective research is needed to confirm our findings. Second, the participants in this study showed relatively more FNOs than generally known poor outcomes. Quite a few patients with poor grades were transferred to another hospital because the patient’s guardians gave up aggressive treatment, such as emergency surgery or intervention. A selection bias may have weakened the power of the results. Third, the exclusion of DCI as a confounding factor for aSAH outcomes is a significant limitation of this study. The diagnosis of DCI requires the occurrence of focal neurologic impairment or a decrease of at least 2 points on the GCS that lasts for at least 1 hour. However, DCI may not be clinically discernable in patients with already poor grade or secondary worsening neurologic status including infection, seizures, respiratory failure, or electrolyte disturbances. Data on the neurological changes of patients were not sufficiently collected owing to retrospective nature of this study. Thus, we tried to analyze the relationship between OER and the CT scan findings after aneurysmal repair. Fourth, the guidelines recommend identifying neurological outcomes 3 months after discharge [9]. However, we only assessed the neurological outcomes at hospital discharge and did not assess the long-term outcomes.
Notes
References
1. Alhoobi M, Abu-Qadous F, Khan M, Shaaban A, Shaikh N, Hammadi F, et al. Ten years’ experiences in the treatment of nonaneurysmal subarachnoid hemorrhage: a retrospective analysis of outcome parameters in a single-center study. Asian J Neurosurg. 15:315–321. 2020.

2. Anderson RC, Kan P, Klimo P, Brockmeyer DL, Walker ML, Kestle JR. Complications of intracranial pressure monitoring in children with head trauma. J Neurosurg. 101(1 Suppl):53–58. 2004.

3. Baggiani M, Graziano F, Rebora P, Robba C, Guglielmi A, Galimberti S, et al. Intracranial pressure monitoring practice, treatment, and effect on outcome in aneurysmal subarachnoid hemorrhage. Neurocrit Care. 38:741–751. 2023.

4. Cho BI, Lee H, Shin H, Kim C, Choi HJ, Kang BS. The prognostic value of optic nerve sheath diameter/eyeball transverse diameter ratio in the neurological outcomes of out-of-hospital cardiac arrest patients. Medicina (Kaunas). 58:1233. 2022.

5. Cho WS, Kim JE, Park SQ, Ko JK, Kim DW, Park JC, et al. Korean clinical practice guidelines for aneurysmal subarachnoid hemorrhage. J Korean Neurosurg Soc. 61:127–166. 2018.

6. de Oliveira Manoel AL, Goffi A, Marotta TR, Schweizer TA, Abrahamson S, Macdonald RL. The critical care management of poor-grade subarachnoid haemorrhage. Crit Care. 20:21. 2016.

7. Dubourg J, Javouhey E, Geeraerts T, Messerer M, Kassai B. Ultrasonography of optic nerve sheath diameter for detection of raised intracranial pressure: a systematic review and meta-analysis. Intensive Care Med. 37:1059–1068. 2011.

8. Geeraerts T, Duranteau J, Benhamou D. Ocular sonography in patients with raised intracranial pressure: the papilloedema revisited. Crit Care. 12:150. 2008.

9. Geocadin RG, Callaway CW, Fink EL, Golan E, Greer DM, Ko NU, et al. Standards for studies of neurological prognostication in comatose survivors of cardiac arrest: a scientific statement from the American Heart Association. Circulation. 140:e517–e542. 2019.

10. Hayashi M, Marukawa S, Fujii H, Kitano T, Kobayashi H, Yamamoto S. Intracranial hypertension in patients with ruptured intracranial aneurysm. J Neurosurg. 46:584–590. 1977.
11. Kang DH, Park J, Lee SH, Park SH, Kim YS, Hamm IS. Does nonperimesencephalic type non-aneurysmal subarachnoid hemorrhage have a benign prognosis? J Clin Neurosci. 16:904–908. 2009.

12. Karnchanapandh K. Effect of increased ICP and decreased CPP on DND and outcome in ASAH. Acta Neurochir Suppl. 114:339–342. 2012.

13. Kim DH, Jun JS, Kim R. Measurement of the optic nerve sheath diameter with magnetic resonance imaging and its association with eyeball diameter in healthy adults. J Clin Neurol. 14:345–350. 2018.

14. Kim DH, Jun JS, Kim R. Ultrasonographic measurement of the optic nerve sheath diameter and its association with eyeball transverse diameter in 585 healthy volunteers. Sci Rep. 7:15906. 2017.

15. Kim JG, Kim W, Shin H, Lim TH, Jang BH, Cho Y, et al. Optic nerve sheath diameter for predicting outcomes in post-cardiac arrest syndrome: an updated systematic review and meta-analysis. J Pers Med. 12:500. 2022.

16. Lee S, Kim YO, Baek JS, Ryu JA. The prognostic value of optic nerve sheath diameter in patients with subarachnoid hemorrhage. Crit Care. 23:65. 2019.

17. Osgood ML. Aneurysmal subarachnoid hemorrhage: review of the pathophysiology and management strategies. Curr Neurol Neurosci Rep. 21:50. 2021.

18. Robba C, Santori G, Czosnyka M, Corradi F, Bragazzi N, Padayachy L, et al. Optic nerve sheath diameter measured sonographically as noninvasive estimator of intracranial pressure: a systematic review and meta-analysis. Intensive Care Med. 44:1284–1294. 2018.

19. Sekhon MS, Griesdale DE, Robba C, McGlashan N, Needham E, Walland K, et al. Optic nerve sheath diameter on computed tomography is correlated with simultaneously measured intracranial pressure in patients with severe traumatic brain injury. Intensive Care Med. 40:1267–1274. 2014.

21. Sheth S, Branstetter BF 4th, Escott EJ. Appearance of normal cranial nerves on steady-state free precession MR images. Radiographics. 29:1045–1055. 2009.

22. Taylor CJ, Robertson F, Brealey D, O’shea F, Stephen T, Brew S, et al. Outcome in poor grade subarachnoid hemorrhage patients treated with acute endovascular coiling of aneurysms and aggressive intensive care. Neurocrit Care. 14:341–347. 2011.

Fig. 1.
Patient selection. SAH : subarachnoid hemorrhage, CPC : cerebral performance category, aSAH : aneurysmal subarachnoid hemorrhage.
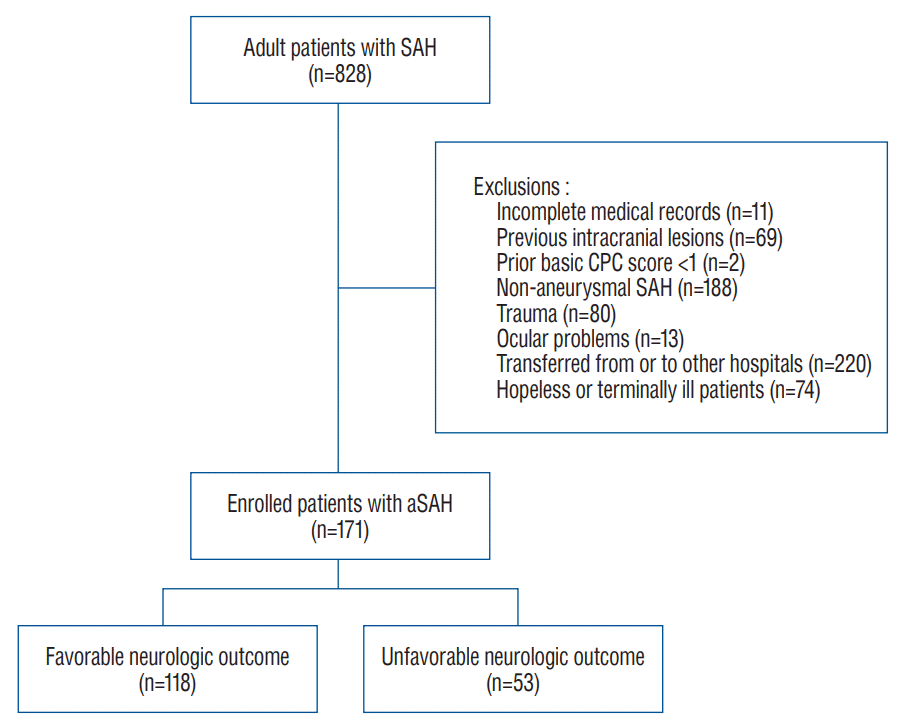
Fig. 2.
Receiver operator curve for predicting unfavorable neurological outcome using ONSD/ETD ratio (OER). AUC was 0.603 (95% confidence interval, 0.515–0.691; p=0.031). ONSD : optic nerve sheath diameter, ETD : eyeball transverse diameter, AUC : area under the receiver operating characteristics curve.

Table 1.
Patient characteristics
Values are presented as mean±standard deviation or number (%). FNO : favorable neurologic outcome, UNO : unfavorable neurologic outcome, BMI : body mass index, HTN : hypertension, DM : diabetes mellitus, ACS : acute coronary syndrome, LC : liver cirrhosis, CKD : chronic kidney disease, GCS : glasgow coma scale, WFNS : World Federation of Neurological Surgeons, CT : computed tomography, OP : operation
Table 2.
Computed tomography scan findings of included patients
| Total (n=171) | FNO (n=118) | UNO (n=53) | p-value | |
|---|---|---|---|---|
| Fisher scale | <0.001 | |||
| 1 | 5 (2.9) | 5 (4.2) | 0 (0.0) | |
| 2 | 12 (7.0) | 10 (8.5) | 2 (3.8) | |
| 3 | 58 (33.9) | 49 (41.5) | 9 (17.0) | |
| 4 | 96 (56.1) | 54 (45.8) | 42 (79.2) | |
| Location of aneurysm | 0.078 | |||
| Anterior cerebral artery | 12 (7.0) | 10 (8.5) | 2 (3.8) | |
| Anterior communicating artery | 51 (29.7) | 37 (31.4) | 14 (26.4) | |
| Basilar artery | 5 (2.9) | 4 (3.4) | 1 (1.9) | |
| Choroidal artery | 3 (1.7) | 1 (0.8) | 2 (3.8) | |
| Middle cerebral artery | 47 (27.3) | 28 (23.7) | 19 (35.8) | |
| Internal carotid artery | 10 (5.8) | 4 (3.4) | 6 (11.3) | |
| Posterior cerebral artery | 1 (0.6) | 0 (0.0) | 1 (1.9) | |
| Posterior communicating artery | 39 (22.7) | 32 (27.1) | 7 (13.2) | |
| Posterior inferior cerebellar artery | 2 (1.2) | 1 (0.8) | 1 (1.9) | |
| Vertebral artery | 1 (0.6) | 1 (0.8) | 0 (0.0) | |
| Hydrocephalus | 67 (39.2) | 36 (30.5) | 31 (58.5) | 0.001 |
| Intraventricular hemorrhage | 87 (50.9) | 46 (39.0) | 41 (77.4) | <0.001 |
| ONSD (mm) | 4.78±0.54 | 4.73±0.57 | 4.89±0.46 | 0.075 |
| ETD (mm) | 23.12±1.06 | 23.16±1.05 | 23.01±1.07 | 0.403 |
| ONSD/ETD ratio | 0.207±0.025 | 0.205±0.026 | 0.213±0.022 | 0.045 |
| Post-CT* | 148 | 107 | 41 | |
| Cerebral edema | 56 (37.8) | 31 (26.3) | 25 (61.0) | 0.007 |
| Cerebral infarction | 34 (23.0) | 19 (17.7) | 15 (36.6) | 0.065 |
| Cerebral ischemia | 38 (25.7) | 29 (27.1) | 9 (22.0) | 0.269 |
| Vasospasm | 22 (14.9) | 14 (13.1) | 8 (19.5) | 0.560 |
Table 3.
Multivariate logistic regression analysis for unfavorable neurological outcomes with baseline variables and optic nerve sheath diameter/eyeball transverse diameter ratio in subarachnoid hemorrhage patients




 PDF
PDF Citation
Citation Print
Print



 XML Download
XML Download