Abstract
Pancreatic cancer, one of the diseases of the elderly, has dismal prognosis, demanding major surgery with high risk and life quality problems, especially in the elderly. Therefore, treatment selection, whether or not to undergo surgery, preoperative risk assessment, and perioperative management of the elderly are becoming critical issues. Although the elderly are expected to have higher morbidity and mortality and lower long-term survival outcomes, surgery is becoming safer over time. Appropriate surgical indication selection, patient-centered decision-making, adequate prehabilitation and postoperative geriatric care are expected to improve surgical outcomes in the elderly. Surgeons must have the concept of geriatric care, and efforts based on institutional systems and academic societies are required. If well selected and prepared, the same surgical principle as non-elderly patients can be applied to elderly patients. In this paper, the surgical treatment of elderly patients with pancreatic cancer is reviewed.
The elderly population is dramatically increasing in most countries, and exponential growth is expected in some countries, including South Korea [1]. As a result, most elderly diseases are on the rise. Pancreatic cancer is one of those diseases. More than 50% of pancreatic cancer patients in Korea are over the age of 70 years, and nearly 20% are over the age of 80 years [2]. Surgeons will see more and more elderly patients with pancreatic cancer. This trend stems from an extended life span.
Pancreatic cancer has dismal prognosis, demanding major surgery with high risk and life quality problems, especially in the elderly. Therefore, treatment selection, whether or not to undergo surgery, preoperative risk assessment, and perioperative management of the elderly are becoming critical issues. In this paper, the surgical treatment of elderly patients with pancreatic cancer is reviewed.
In a large population-based cohort study, early outcomes after pancreatectomy including mortality worsened with age, and the odds ratio of in-hospital mortality was remarkable in those aged 80 years or older [3]. A national study in the United States using a nationwide inpatient sample reported a higher surgical mortality rate for octogenarian patients [4]. Cancer registry data from the Netherlands also demonstrated an increasing mortality rate with advancing age after pancreatic cancer surgery; however, the long-term survival benefit was acceptable despite a slight decline in the elderly [5]. A Chinese study also showed that pancreatic cancer-specific survival by American Joint Committee on Cancer (AJCC) stage and Surveillance, Epidemiology, and End Results (SEER) stage was negatively correlated with age [6]. Studies in Japan and Korea demonstrated better outcomes after surgery than nonsurgical treatment in potentially resectable cancer in the elderly [78]. According to data from Seoul National University, once resection was performed, there was no difference in the cumulative recurrence rate in the elderly [9].
A recent systematic review summarized the outcomes of surgery for pancreatic cancer in the elderly [10]. In the enrolled studies, the age definition of the elderly was elevated from the 70s to the 80s over time. The authors compared surgical mortality, complications, and overall survival between elderly and non-elderly adults during the 2 time periods starting study enrollment before and after 2000. Mortality rate of older adults improved and became the same as in younger adults, while complications and survival rates improved but still had a worse prognosis in older adults.
An analysis of data from the United States National Cancer Database on 98 pancreatic cancer patients over the age of 90 showed high mortality and low survival rates. In addition, chemoradiation alone showed similar survival results with surgery alone [11].
In summary, morbidity and mortality are higher in the elderly, but the gap is narrowing. It would be difficult to explain how surgery has become safer over time for elderly patients with pancreatic cancer. Improvement of perioperative and operative care as well as general health status of the elderly would be the important reasons. Long-term survival in older people is generally worse, but surgical treatment offers a better chance than other treatment modalities. Surgical resection may benefit appropriately selected elderly patients with pancreatic cancer.
Surgeons must appropriately select surgical candidates and decide on neoadjuvant therapy (NAT) vs. upfront surgery. Both host and disease factors must be considered for decision-making. A United States national study found that patients older than 80 years had worse survival outcomes, particularly those with 2 or more comorbid conditions [4]. A study from the National Cancer Center, which found that the overall survival rate after surgery was significantly higher in elderly patients with resectable pancreatic cancer compared to nonsurgical treatment, also revealed that the comorbidity index was an independent factor in the overall survival rate [7]. Satoi and colleagues [8] showed that overall survival in patients with potentially resectable cancer in their 80s was better only in mentally and physically fit older patients with favorable operative risk scores. Therefore, the risk of surgery must be assessed to detect impairment not identified on routine examination and to predict short-term outcome and overall survival so that appropriate treatment can be selected. For this purpose, a comprehensive geriatric assessment is strongly recommended to develop a treatment plan [12].
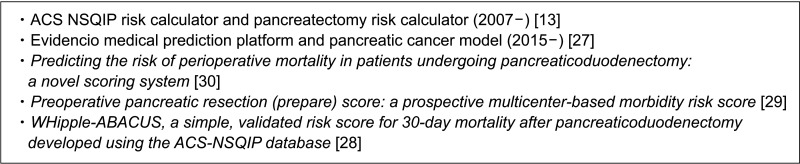
Fig. 1 shows risk assessment scales, indices, or scoring systems used in practice [1314151617181920212223242526]. Fig. 2 shows some samples of general or specific risk scoring systems for predicting pancreatectomy morbidity and mortality, and there are different scoring systems for predicting specific complications [1327282930]. The International Society of Geriatric Oncology established a consensus on geriatric assessment in older patients before treatment [12]. The domains to be measured and some tools are summarized in Table 1 [12]. Winer and Dotan [31] explored relevance in geriatric domains including functional, nutritional, and psychological status, social support, comorbidity, polypharmacy, cognition, and prediction of chemotherapy toxicity for pancreatic cancer, which was mostly about tolerability and effects on treatment modalities.
As one of the disease factors, decisions should be made considering the extent of the disease and resectability. Kondo et al. [32] reported that patients older than 80 years of age had a similar prognosis for resectable pancreatic cancer, but a worse prognosis for borderline or locally advanced cancer than those younger than 80 years of age. A multicenter study in Japan showed poor long-term outcomes in the elderly, especially in borderline resectable cancer, and suggested that the poorer survival rates in the elderly were due to lower adjuvant therapy completion rates [33]. A study by the European group RESPECT reported that elderly patients treated with NAT had comparable resectability and survival rates to that of younger borderline resectable and locally advanced pancreatic cancer patients [34].
As age is a major risk factor for dropout in treatment after surgery, NAT might provide a greater chance to complete adjuvant therapy for the elderly. Recent studies by some influential groups have consistently reported that NAT is safe and effective for elderly patients with resectable pancreatic cancer [35363738]. Randomized clinical trials for NAT are recommended in elderly pancreatic cancer patients who are considered tolerable to both surgery and chemotherapy.
The next step is decision-making. Surgery in the elderly can lead to unwanted burdens and unintended consequences such as loss of function and reduced quality of life. The elderly may have different values, goals, and preferences. In addition, there is a lack of high-quality evidence to interpret the results of preoperative risk assessment in geriatric patients, not only because there is no objective cutoff in preoperative evaluation criteria, but also because geriatric patients are under-represented in clinical trials. Thus, traditional ‘informed consent’ is not sufficient. A patient-centered approach using shared decision-making has been proposed to integrate the patient’s preferences, values, and goals with underlying health conditions so that patients and physicians can make decisions together. Potential outcomes of shared decision-making include reduction of unwanted and aggressive treatment, fewer symptoms of postoperative stress, and value concordant decisions [38].
Once the decision is made to proceed with surgery, preoperative, operative, and postoperative geriatric-specific management should be considered. The purpose of prehabilitation, which is likely to be most useful for older cancer patients, is to help prepare the patient for surgery in order to reduce recovery time and postoperative complications. The core components are cardiovascular and skeletal muscle training, nutritional management, psychological support, and medical optimization. The benefits of prehabilitation have been seen in some major surgeries but have not been clear in pancreatic surgeries. However, pancreatic cancer often has modifiable risk factors associated with significant nutritional impairments such as cachexia and sarcopenia, which are potential targets for prehabilitation. Therefore, elderly patients with pancreatic cancer may benefit the most from a prehabilitation program to improve nutritional status as well as cardiorespiratory function.
According to a recent systematic review and meta-analysis on minimally invasive pancreaticoduodenectomy in the elderly, minimally invasive surgery is safe, feasible, and can be used as a potential alternative to open surgery. Although minimally invasive surgery cannot eliminate a high risk of mortality and morbidity, it is recommended because it is less painful, and early recovery is very important for the elderly [3940].
How about vascular resection? Vein resection does not appear to cause significantly higher morbidity, and survival outcomes are comparable for younger patients [4142]. However, in cases of arterial resection, there are only a few reports on successful experiences of celiac axis resection in the elderly [4344].
After surgery, postoperative care specific to geriatric patients should be performed. Fig. 3 is a list of postoperative geriatric care requiring a multidisciplinary approach. Whether enhanced recovery after surgery (ERAS) is effective for pancreatectomy and applicable in the elderly remains a question to be answered. According to the updated ERAS guidelines and meta-analysis, although there are some favorable reports on the benefits of ERAS, new randomized controlled trials with low risk of bias are needed to provide evidence of the effectiveness of ERAS in pancreatic surgery [4546]. Thus, at this point, ERAS may be particularly important for older patients and/or less physically fit patients.
Of the short-term outcome measures of ERAS, at least the length of hospital stay after pancreaticoduodenectomy decreased in elderly patients [47]. Although prehabilitation and ERAS are clearly beneficial for the elderly, a tailored multimodal prehabilitation and ERAS program for elderly patients based on evidence-based cutoff values is needed [48].
Therefore, the same operating principle can be applied to the elderly if properly selected. However, adding surgical risk through extensive surgery is not recommended, especially in very elderly patients with localized advanced disease. Tailored perioperative care may improve operative outcomes in elderly patients.
Some countries have good institutional system models for geriatric surgical care. For example, the Dukes Center for Geriatric Surgery’s Perioperative Optimization of Senior Health system seems well established. As a result of using this system, it was reported that the length of hospitalization, readmission rate, and complication rate decreased, indicating that the institutional system is working properly [49].
Only 7% of randomized controlled trials are specifically designed for the elderly. Elderly pancreatic cancer patients have been under-represented in clinical trials because the elderly group in clinical trials is much smaller than the actual cancer population. As a result, it is not possible to simply extrapolate clinical trial data to the elderly [50]. Therefore, clinical trials designed specifically for the elderly with pancreatic cancer are needed.
Among several clinical practice guidelines, only the Japan Pancreas Society guidelines raised the age-specific question of whether surgical treatment is recommended for pancreatic cancer patients aged 80 years or older. Their statement was “surgical treatment may be considered for elderly patients with pancreatic cancer 80 years or older if they wish to undergo surgery and their general condition allows it” [51]. Although it seems obscure, it describes all the important factors, the patient’s wishes, and risk assessment.
In 2019, experts in geriatric oncology and hepato-biliary-pancreatic surgery met to conduct a state-of-the-art overview of multimodality approaches and explore the latest clinical pathways for geriatric patients with hepato-biliary-pancreatic malignancies. The results of this study were published in a special issue of the European Journal of Surgical Oncology [52].
Although the elderly are expected to have higher morbidity and mortality and lower long-term survival outcomes, surgery is becoming safer over time. Appropriate surgical indication selection, patient-centered decision-making, adequate prehabilitation, and postoperative geriatric care including ERAS are expected to improve surgical outcomes in the elderly. Surgeons must have the concept of geriatric care, and efforts based on institutional systems and academic societies are required. If well selected and prepared, the same surgical principle as non-elderly patients can be applied to elderly patients.
References
1. Population Division, Department of Economic and Social Affairs, United Nations. World population prospects: the 2008 revision. Volume II. Sex and age distribution of the world population. United Nations Publication;2009. Available from: https://www.un.org/development/desa/pd/sites/www.un.org.development.desa.pd/files/files/documents/2020/Jan/un_2008_world_population_prospects-2008_revision_volume-ii.pdf
.
2. Kang MJ, Lim J, Han SS, Park HM, Park SJ, Won YJ, et al. First course of treatment and prognosis of exocrine pancreatic cancer in Korea from 2006 to 2017. Cancer Res Treat. 2022; 54:208–217. PMID: 34030432.

3. Riall TS, Reddy DM, Nealon WH, Goodwin JS. The effect of age on short-term outcomes after pancreatic resection: a population-based study. Ann Surg. 2008; 248:459–467. PMID: 18791366.
4. Finlayson E, Fan Z, Birkmeyer JD. Outcomes in octogenarians undergoing high-risk cancer operation: a national study. J Am Coll Surg. 2007; 205:729–734. PMID: 18035254.

5. van der Geest LG, Besselink MG, van Gestel YR, Busch OR, de Hingh IH, de Jong KP, et al. Pancreatic cancer surgery in elderly patients: balancing between short-term harm and long-term benefit: a population-based study in the Netherlands. Acta Oncol. 2016; 55:278–285. PMID: 26552841.

6. Wang H, Liu J, Xia G, Lei S, Huang X, Huang X. Survival of pancreatic cancer patients is negatively correlated with age at diagnosis: a population-based retrospective study. Sci Rep. 2020; 10:7048. PMID: 32341400.

7. Park HM, Park SJ, Han SS, Kim SH. Surgery for elderly patients with resectable pancreatic cancer, a comparison with non-surgical treatments: a retrospective study outcomes of resectable pancreatic cancer. BMC Cancer. 2019; 19:1090. PMID: 31718565.

8. Satoi S, Yamamoto T, Uchida K, Fujii T, Kin T, Hirano S, et al. Optimal treatment for octogenarians with resectable and borderline resectable pancreatic ductal adenocarcinoma: a multicenter retrospective study. Pancreas. 2020; 49:837–844. PMID: 32590619.

9. Kang JS, Kim H, Kim JR, Han Y, Kim E, Byun Y, et al. Short- and long-term outcomes of pancreaticoduodenectomy in elderly patients with periampullary cancer. Ann Surg Treat Res. 2020; 98:7–14. PMID: 31909045.

10. Tan E, Song J, Lam S, D’Souza M, Crawford M, Sandroussi C. Postoperative outcomes in elderly patients undergoing pancreatic resection for pancreatic adenocarcinoma: a systematic review and meta-analysis. Int J Surg. 2019; 72:59–68. PMID: 31580919.

11. Meltzer RS, Kooby DA, Switchenko JM, Datta J, Carpizo DR, Maithel SK, et al. Does major pancreatic surgery have utility in nonagenarians with pancreas cancer? Ann Surg Oncol. 2021; 28:2265–2272. PMID: 33141373.

12. Wildiers H, Heeren P, Puts M, Topinkova E, Janssen-Heijnen ML, Extermann M, et al. International Society of Geriatric Oncology consensus on geriatric assessment in older patients with cancer. J Clin Oncol. 2014; 32:2595–2603. PMID: 25071125.

13. American College of Surgeons (ACS). ACS NSQIP Surgical risk calculator [Internet]. ACS National Surgical Quality Improvement Program;c2007-2023. cited 2022 Dec 1. Available from: https://riskcalculator.facs.org/RiskCalculator/PatientInfo.jsp
.
14. Oken MM, Creech RH, Tormey DC, Horton J, Davis TE, McFadden ET, et al. Toxicity and response criteria of the Eastern Cooperative Oncology Group. Am J Clin Oncol. 1982; 5:649–655. PMID: 7165009.

15. Karnofsky DA, Abelmann WH, Craver LF, Burchenal JH. The use of the nitrogen mustards in the palliative treatment of carcinoma: with particular reference to bronchogenic carcinoma. Cancer. 1948; 1:634–656.

16. Copeland GP, Jones D, Walters M. POSSUM: a scoring system for surgical audit. Br J Surg. 1991; 78:355–360. PMID: 2021856.

17. Rockwood K, Song X, MacKnight C, Bergman H, Hogan DB, McDowell I, et al. A global clinical measure of fitness and frailty in elderly people. CMAJ. 2005; 173:489–495. PMID: 16129869.

18. Shahrokni A, Tin A, Alexander K, Sarraf S, Afonso A, Filippova O, et al. Development and evaluation of a new frailty index for older surgical patients with cancer. JAMA Netw Open. 2019; 2:e193545. PMID: 31074814.

19. Kashani KB, Frazee EN, Kukrálová L, Sarvottam K, Herasevich V, Young PM, et al. Evaluating muscle mass by using markers of kidney function: development of the sarcopenia index. Crit Care Med. 2017; 45:e23–e29. PMID: 27611976.

20. Janssen I, Heymsfield SB, Ross R. Low relative skeletal muscle mass (sarcopenia) in older persons is associated with functional impairment and physical disability. J Am Geriatr Soc. 2002; 50:889–896. PMID: 12028177.

21. Bouillanne O, Morineau G, Dupont C, Coulombel I, Vincent JP, Nicolis I, et al. Geriatric Nutritional Risk Index: a new index for evaluating at-risk elderly medical patients. Am J Clin Nutr. 2005; 82:777–783. PMID: 16210706.

22. Charlson ME, Pompei P, Ales KL, MacKenzie CR. A new method of classifying prognostic comorbidity in longitudinal studies: development and validation. J Chronic Dis. 1987; 40:373–383. PMID: 3558716.

23. Piccirillo JF, Creech CM, Zequeira R, Anderson S, Johnston AS. Inclusion of comorbidity into oncology data registries. J Regist Manag. 1999; 26:66–70.
24. van Walraven C, Austin PC, Jennings A, Quan H, Forster AJ. A modification of the Elixhauser comorbidity measures into a point system for hospital death using administrative data. Med Care. 2009; 47:626–633. PMID: 19433995.

25. Saliba D, Orlando M, Wenger NS, Hays RD, Rubenstein LZ. Identifying a short functional disability screen for older persons. J Gerontol A Biol Sci Med Sci. 2000; 55:M750–M756. PMID: 11129398.

26. Haga Y, Ikei S, Ogawa M. Estimation of Physiologic Ability and Surgical Stress (E-PASS) as a new prediction scoring system for postoperative morbidity and mortality following elective gastrointestinal surgery. Surg Today. 1999; 29:219–225. PMID: 10192731.

27. Evidencio Medical Decision Support. Risk of complications in patients following pancreaticoduodenectomy [Internet]. Evidencio Community;2018. cited 2022 Dec 1. Available from: https://www.evidencio.com/models/show/1198
.
28. Gleeson EM, Shaikh MF, Shewokis PA, Clarke JR, Meyers WC, Pitt HA, et al. WHipple-ABACUS, a simple, validated risk score for 30-day mortality after pancreaticoduodenectomy developed using the ACS-NSQIP database. Surgery. 2016; 160:1279–1287. PMID: 27544541.

29. Uzunoglu FG, Reeh M, Vettorazzi E, Ruschke T, Hannah P, Nentwich MF, et al. Preoperative Pancreatic Resection (PREPARE) score: a prospective multicenter-based morbidity risk score. Ann Surg. 2014; 260:857–864. PMID: 25243549.
30. Venkat R, Puhan MA, Schulick RD, Cameron JL, Eckhauser FE, Choti MA, et al. Predicting the risk of perioperative mor tal it y in pat ients undergoing pancreaticoduodenectomy: a novel scoring system. Arch Surg. 2011; 146:1277–1284. PMID: 22106320.

31. Winer A, Dotan E. Treatment paradigms for older adults with pancreatic cancer: a nuanced approach. Curr Treat Options Oncol. 2021; 22:104. PMID: 34596801.

32. Kondo N, Uemura K, Nakagawa N, Okada K, Seo S, Takahashi S, et al. Reappraisal of the validity of surgery for patients with pancreatic cancer aged 80 years or older stratified by resectability status. J Hepatobiliary Pancreat Sci. 2020; 27:64–74. PMID: 31602815.

33. Sho M, Murakami Y, Kawai M, Motoi F, Satoi S, Matsumoto I, et al. Prognosis after surgical treatment for pancreatic cancer in patients aged 80 years or older: a multicenter study. J Hepatobiliary Pancreat Sci. 2016; 23:188–197. PMID: 26763744.

34. Weniger M, Moir J, Damm M, Maggino L, Kordes M, Rosendahl J, et al. Neoadjuvant therapy in elderly patients receiving FOLFIRINOX or gemcitabine/nab-paclitaxel for borderline resectable or locally advanced pancreatic cancer is feasible and lead to a similar oncological outcome compared to non-aged patients: results of the RESPECT-Study. Surg Oncol. 2020; 35:285–297. PMID: 32949968.

35. Rieser CJ, Zenati M, Narayanan S, Bahary N, Lee KK, Paniccia A, et al. Optimal management of resectable pancreatic head cancer in the elderly patient: does neoadjuvant therapy offer a survival benefit? Ann Surg Oncol. 2021; 28:6264–6272. PMID: 33748894.

36. Rieser CJ, Narayanan S, Bahary N, Bartlett DL, Lee KK, Paniccia A, et al. Optimal management of patients with operable pancreatic head cancer: a Markov decision analysis. J Surg Oncol. 2021; 124:801–809. PMID: 34231222.

37. Cooper AB, Holmes HM, des Bordes JK, Fogelman D, Parker NH, Lee JE, et al. Role of neoadjuvant therapy in the multimodality treatment of older patients with pancreatic cancer. J Am Coll Surg. 2014; 219:111–120. PMID: 24856952.

38. Chesney TR, Schwarze ML. Patient-centered surgical decision making. Rosenthal RA, Zenilman ME, Katlic MR, editors. Principles and practice of geriatric surgery. Springer International Publishing;2020. p. 81–93.
39. Zhang W, Huang Z, Zhang J, Che X. Effect of laparoscopic pancreaticoduodenectomy in elderly people: a meta-analysis. Pancreas. 2021; 50:1154–1162. PMID: 34714278.

40. Zhu J, Wang G, Du P, He J, Li Y. Minimally invasive pancreaticoduodenectomy in elderly patients: systematic review and meta-analysis. World J Surg. 2021; 45:1186–1201. PMID: 33458781.

41. Kanda M, Fujii T, Suenaga M, Takami H, Inokawa Y, Yamada S, et al. Pancreatoduodenectomy with portal vein resection is feasible and potentially beneficial for elderly patients with pancreatic cancer. Pancreas. 2014; 43:951–958. PMID: 24717827.

42. Fang JZ, Lu CD, Wu SD, Huang J, Zhou J. Portal vein/superior mesenteric vein resection in pancreatic cancer treatment in the elderly. Medicine (Baltimore). 2017; 96:e7335. PMID: 28682880.

43. Jegatheeswaran S, Baltatzis M, Jamdar S, Siriwardena AK. Superior mesenteric artery (SMA) resection during pancreatectomy for malignant disease of the pancreas: a systematic review. HPB (Oxford). 2017; 19:483–490. PMID: 28410913.

44. Wang X, Dong Y, Jin J, Liu Q, Zhan Q, Chen H, et al. Efficacy of modified Appleby surgery: a benefit for elderly patients? J Surg Res. 2015; 194:83–90. PMID: 25311939.

45. Melloul E, Lassen K, Roulin D, Grass F, Perinel J, Adham M, et al. Guidelines for perioperative care for pancreatoduodenectomy: enhanced recovery after surgery (ERAS) recommendations 2019. World J Surg. 2020; 44:2056–2084. PMID: 32161987.

46. Ji HB, Zhu WT, Wei Q, Wang XX, Wang HB, Chen QP. Impact of enhanced recovery after surgery programs on pancreatic surgery: a meta-analysis. World J Gastroenterol. 2018; 24:1666–1678. PMID: 29686474.
47. Raza SS, Nutu OA, Powell-Brett S, Carvalheiro Boteon A, Hodson J, Abradelo M, et al. Impact of an enhanced recovery after surgery protocol on short-term outcomes in elderly patients undergoing pancreaticoduodenectomy. HPB (Oxford). 2022; 24:1720–1728. PMID: 35643908.
48. Bongers BC, Dejong CH, den Dulk M. Enhanced recovery after surgery programmes in older patients undergoing hepatopancreatobiliary surgery: what benefits might prehabilitation have? Eur J Surg Oncol. 2021; 47(3 Pt A):551–559. PMID: 32253075.
49. McDonald SR, Heflin MT, Whitson HE, Dalton TO, Lidsky ME, Liu P, et al. Association of integrated care coordination with postsurgical outcomes in high-risk older adults: the Perioperative Optimization of Senior Health (POSH) Initiative. JAMA Surg. 2018; 153:454–462. PMID: 29299599.

50. Talarico L, Chen G, Pazdur R. Enrollment of elderly patients in clinical trials for cancer drug registration: a 7-year experience by the US Food and Drug Administration. J Clin Oncol. 2004; 22:4626–4631. PMID: 15542812.

51. Okusaka T, Nakamura M, Yoshida M, Kitano M, Uesaka K, Ito Y, et al. Clinical practice guidelines for pancreatic cancer 2019 from the Japan Pancreas Society: a synopsis. Pancreas. 2020; 49:326–335. PMID: 32132516.

52. Backen A, Lamarca A, Hubner RA, McNamara MG, Valle JW. HPB cancers in older patients: inclusion of older/senior patients in clinical trials. Eur J Surg Oncol. 2021; 47(3 Pt A):597–602. PMID: 33298342.

Fig. 1
Preoperative risk assessment. ASA, American Society of Anesthesiology; ECOG, Eastern Cooperative Oncology Group; POSSIUM, Physiologic and Operative Severity Score for the enumeration of Mortality and Morbidity; CSHA, The Canadian Study of Health and Aging; CFS, clinical frailty scale; MSKFI, Memorial Sloan Kettering frailty index; SMI, Skeletal Muscle Index; GNRI, Geriatric Nutritional Risk Index; VES-13, The Vulnerable Elders Survey; E-PASS, Estimation of Physiologic Ability and Surgical Stress.
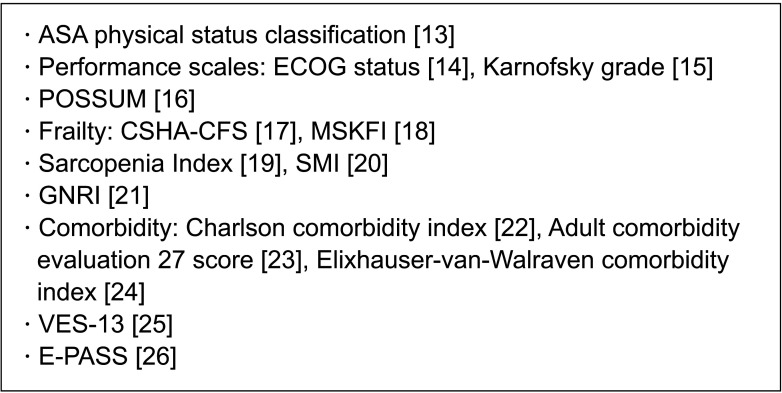
Table 1
Geriatric assessment domains and tools
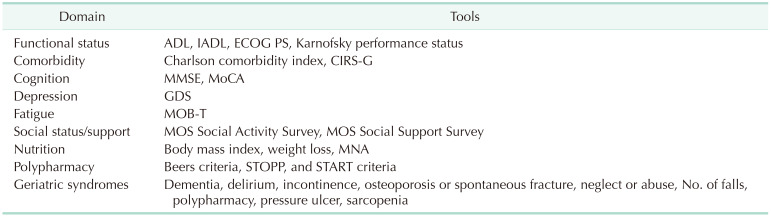
ADL, activity of daily living; IADL, instrumental activity of daily living; ECOG, Eastern Cooperative Oncology Group; PS, performance status; CIRS-G, Cumulative Illness Rating Scale-Geriatrics; MMSE, mini-mental state examination; MoCA, Montreal Cognitive Assessment; GDS, Geriatric Depression Scale; MOB-T, Mobility Tiredness Test; MOS, Medical Outcomes Study; MNA, mini nutritional assessment; STOPP, Screening Tool of Older Person’s Prescriptions; START, Screening Tool to Alert Doctors to Right Treatment.




 PDF
PDF Citation
Citation Print
Print




 XML Download
XML Download