Abstract
Background
Hürthle cell carcinoma (HCC), a type of thyroid carcinoma, is rare in South Korea, and few studies have investigated its prognosis.
Methods
This long-term multicenter retrospective cohort study evaluated the clinicopathological features and clinical outcomes in patients with HCC who underwent thyroid surgery between 1996 and 2009.
Results
The mean age of the 97 patients included in the study was 50.3 years, and 26.8% were male. The mean size of the primary tumor was 3.2±1.8 cm, and three (3.1%) patients had distant metastasis at initial diagnosis. Ultrasonographic findings were available for 73 patients; the number of nodules with low-, intermediate-, and high suspicion was 28 (38.4%), 27 (37.0%), and 18 (24.7%), respectively, based on the Korean-Thyroid Imaging Reporting and Data System. Preoperatively, follicular neoplasm (FN) or suspicion for FN accounted for 65.2% of the cases according to the Bethesda category, and 13% had malignancy or suspicious for malignancy. During a median follow-up of 8.5 years, eight (8.2%) patients had persistent/recurrent disease, and none died of HCC. Older age, gross extrathyroidal extension (ETE), and widely invasive types of tumors were significantly associated with distant metastasis (all P<0.01). Gross ETE (hazard ratio [HR], 27.7; 95% confidence interval [CI], 2.2 to 346.4; P=0.01) and widely invasive classification (HR, 6.5; 95% CI, 1.1 to 39.4; P=0.04) were independent risk factors for poor disease-free survival (DFS).
Conclusion
The long-term prognosis of HCC is relatively favorable in South Korea from this study, although this is not a nation-wide data, and gross ETE and widely invasive cancer are significant prognostic factors for DFS. The diagnosis of HCC by ultrasonography and cytopathology remains challenging.
Hürthle cell carcinoma (HCC) is a rare thyroid carcinoma that accounts for 3% to 4% of all malignant thyroid tumors [1,2]. Hürthle or oxyphilic cells are usually larger than the normal follicular cells and are characterized by abundant eosinophilic cytoplasm [3]. Thyroid nodules comprising 75% or more Hürthle cells are classified as Hürthle cell neoplasms (HCNs) [4]. Similar to follicular neoplasms (FNs), thyroid tumors demonstrating capsular and/or vascular invasion, lymph node (LN) metastasis, or presence of distant metastasis are diagnosed as HCC [4,5]. Previously, HCC was classified as an oxyphilic variant of follicular thyroid cancers, by the World Health Organization (WHO) [6]. However, due to their distinct genetic expression, unique pathologic characteristics, and clinical behavior, HCCs have been reclassified as a separate subtype of differentiated thyroid cancer (DTC) [7,8].
In general, HCC has demonstrated more aggressive clinical behavior than other DTCs, in terms of more extrathyroidal extension (ETE), distant metastasis, less iodine-avid, and higher rates of mortality [9-11]. A population-level analysis using the Surveillance, Epidemiology, and End Results database reported that patients with HCC were older, presented with larger tumors, and had higher disease-specific mortality than patients with DTC [9]. However, not all studies have demonstrated consistent results [12-14]. As the prevalence of HCC is lower in regions with high iodine intakes, such as South Korea and Japan, limited studies have addressed the prognosis of patients with HCC in South Korea [15,16]. Considering the relatively small number of patients and a short follow-up period in these studies, a study that can fill this gap is necessary.
In addition, preoperative diagnosis of HCC by ultrasonography (US) and fine-needle aspiration (FNA) cytology is challenging. Until now, several studies have reported the imaging and cytopathology findings of HCC [16-18]. However, preoperative US and pathological features have not been elucidated because the number of patients with HCC included in these studies were small, and Hürthle cell adenoma was also included in the analysis.
Therefore, we aimed to evaluate the clinicopathological characteristics and long-term clinical outcomes in patients with HCC in a multi-institutional cohort in South Korea. Furthermore, we evaluated the prognostic factors that might be associated with distant metastasis at the time of diagnosis and persistent/recurrent disease during follow-up in patients with HCC.
From January 1996 to December 2013, a total of 97 patients with HCC underwent thyroid surgery at five tertiary care hospitals in South Korea. All patients included in this retrospective multicenter study were confirmed to have HCC by histopathology and were aged >18 years. The study protocol was approved by the relevant Institutional Review Boards (Asan Medical Center, 2021-0568; Ulsan University Hospital, 2016-12-031; Pusan National University Hospital, 2105-038-013; Chonnam National University Hwasun Hospital, CNUHH-2017-053; and Chungnam National University Hospital, CNUH 2017-01-018). The need for informed consent was waived due to the retrospective design of the study.
The findings on the preoperative US of the neck were retrospectively reviewed by an experienced endocrinologist and radiologist at each center. US findings of the target nodule were evaluated according to the Korean Thyroid Imaging Reporting and Data System (K-TIRADS) [19]. The internal content of the nodule was categorized according to the ratio of the cystic to the solid portion within a nodule (e.g., predominantly solid, >10% and ≤50% cystic). Echogenicity of the nodule was defined as a hypoechogenic pattern compared with an echogenicity pattern in the thyroid parenchyma and a marked hypoechogenic pattern as compared with that of the strap muscle. The nodule orientation was categorized as parallel or non-parallel. The tumor margins were classified as well-defined smooth, spiculated, or ill-defined. Calcifications were categorized as microcalcification (calcification foci ≤1 mm), macrocalcification (calcification foci >1 mm), or none. Preoperative cytopathology of the nodule was reported according to the 2017 Bethesda system [20].
After thyroid surgery, subsequent radioactive iodine (RAI) therapy was administered according to guideline and physician’s decision [21,22]. All patients were regularly followed up every 6 to 12 months. Physical examinations such as palpation of the neck and blood investigations, including thyroid function test, serum thyroglobulin, and anti-thyroglobulin antibody levels, were performed at each visit. Neck US was performed every 12 to 24 months, and US-guided FNA cytology was performed when there were abnormal findings on the US. Additional diagnostic imaging studies including neck/chest computed tomography or 18F-fluorodeoxyglucose-positron emission tomography were performed as needed such as increasing serum thyroglobulin [23].
Persistence/recurrence of disease was defined as the appearance of pathologically proven malignant tissue and/or appearance of metastatic lesions. Synchronous distant metastasis was defined as with persistent disease during follow-up. Diseasefree survival (DFS) was defined as the time from the date of surgery until last follow-up or recurrence. Disease-specific mortality was defined as death from HCC, and overall-mortality was defined as death from all causes.
Statistical analyses were performed using the R program version 3.5.1 (R Foundation for Statistical Computing, Vienna, Austria; http://www.R-project.org), and figures were constructed using Graph Pad Prism version 5.0 (GraphPad Software, SanDiego, CA, USA; http://www.graphpad.com). Continuous variables are presented as mean±standard deviation or medians and interquartile ranges (IQRs), and categorical variables are presented as numbers (percentages). We used the t test and Kruskal-Wallis rank sum test to compare continuous variables and chi-square test to compare categorical variables. The Cox proportional hazard model was used to analyze the prognostic factors associated with DFS, presented as hazard ratios (HRs), 95% confidence intervals (CIs), and P values. Statistical significance was set at P<0.05.
Table 1 presents the baseline characteristics of the 97 patients with HCC. The mean age of the patients at the time of initial surgery was 50.3±13.4 years, and 26.8% were males. The mean size of the primary tumor was 3.2±1.8 cm, and 25.8% of the tumors were larger than 4 cm. Microscopic ETE and gross ETE were found in 12 (12.4%) and three (3.1%) patients, respectively. Vascular invasion was found in 24 (24.7%) patients, among whom three had extensive vascular invasion (≥4 foci). Based on the WHO classification, the number of patients with minimally invasive, encapsulated angio-invasive, and widely invasive tumors was 73 (75.3%), 19 (19.6%), and five (5.1%), respectively. At the time of diagnosis, two (2.1%) patients had cervical LN metastasis, and three (3.1%) patients had distant metastasis. Based on the 8th edition of the American Joint Committee on Cancer/Union for International Cancer Control tumornode-metastasis (TNM) staging system, the number of patients with stage I, II, III, and IV classification was 82 (84.5%), 12 (12.4%), 0, and three (3.1%), respectively. Forty-eight (49.5%) patients underwent total thyroidectomy, and 40 of them received subsequent RAI therapy. During a median follow-up of 8.5 years (IQR, 5.2 to 12.8), eight (8.2%) patients had persistent/recurrent disease. Among eight patients, four patients had local recurrence such as operation bed and cervical LN metastasis. One patient had newly detected subcutaneous metastasis and the other three had persistent metastasis in lung and bone. Nine (9.3%) patients died from other causes, and none of them died of HCC.
Of the 97 patients, US findings of HCC were available for 73 patients (Table 2). Most (n=62, 84.9%) were solid nodules, and in terms of echogenicity, 40 (54.8%) were hypoechoic, and nine (12.3%) were markedly hypoechoic. Ten nodules (13.7%) had non-parallel orientation, and 68 (93.2%) had smooth margins. In terms of calcification, seven (9.6%) had microcalcification, and nine (12.3%) had macrocalcification. Based on the K-TIRADS, 28 (38.4%), 27 (37.0%), and 18 (24.7%) patients were classified into the low-, intermediate-, and high suspicion categories, respectively (Fig. 1A).
Among all patients included in the study, results of preoperative FNA or core needle biopsy (CNB) were available for 92 patients. According to the Bethesda category, FN or suspected FN was the most common and was observed in 60 (65.2%) patients (Table 2, Fig. 1B). Atypia of undetermined significance/ follicular lesion of undetermined significance, suspicious for malignancy, and malignancy accounted for 8.7%, 5.4%, and 7.6% of the cases, respectively. The FNA/CNB results according to the K-TIRADS category are shown in Fig. 1C. The proportion of tumors with Bethesda category IV–VI increased toward from low to high suspicion nodule, accounts for 74.1%, 80.8%, and 83.4%, respectively. CNB was performed in 11 out of 92 patients and there was no significant difference between pathology according to FNA or CNB (P=0.24).
We compared the clinicopathological features between patients with and without distant metastasis at initial surgery (Supplemental Table S1). Patients with distant metastasis were significantly older than those without distant metastasis (68.5 years vs. 49.8 years, P<0.01). There was no significant difference in the sex ratio and primary tumor size between the two groups. Two (66.7%) patients in the distant metastasis group had gross ETE, which was significantly higher than that (1.1%) in patients without distant metastasis (P<0.01). According to the WHO classification, widely invasive (66.7%) and encapsulated angio-invasive (33.3%) tumors were observed more frequently in patients with distant metastasis than in those without distant metastasis (P<0.01).
We also evaluated the clinical and ultrasonographic features of patients who had features of high risk of recurrent disease such as gross ETE, LN metastasis, distant metastasis, and widely invasive tumor. Because some factors overlap, there are 9 patients with high-risk of recurrent disease (Supplemental Table S2). They were older (67.5 years vs. 49.8 years, P<0.01) than those with low risk of recurrence and showed a higher rate of total thyroidectomy (88.9% vs. 45.5%, P=0.03).
To identify the clinicopathological factors associated with DFS in patients with HCC, univariate and multivariate analyses were performed (Table 3). Older age (≥55 years, HR, 6.2; 95% CI, 1.2 to 30.9; P=0.03), gross ETE (HR, 82.5; 95% CI, 7.4 to 923.5; P<0.01), and widely invasive tumors according to the WHO classification (HR, 17.3; 95% CI, 3.5 to 85.9; P<0.01) were significantly associated with poorer DFS. In multivariate analysis, gross ETE (HR, 27.7; 95% CI, 2.2 to 346.4; P=0.01) and widely invasive tumors (HR, 6.5; 95% CI, 1.1 to 39.4; P=0.04) were independent risk factors associated with DFS.
HCC is a relatively uncommon thyroid malignancy in iodinesufficient regions. According to a single-center study in South Korea, among patients diagnosed with thyroid cancer during 1995 to 2005, HCC accounted for only 1% [15]. In this multicenter cohort study in South Korea, where HCC is relatively rare, we evaluated the clinical outcome of patients with HCC. Persistent/recurrent disease was observed in 8.2% of the patients, and none died of HCC during a median follow-up of 8.5 years. Distant metastasis was significantly associated with older age, gross ETE, and widely invasive cancer. We found that the clinicopathological factors associated with DFS, gross ETE, and widely invasive cancer were independent risk factors associated with poor DFS.
Previous studies reported that HCC is associated with a more aggressive clinical behavior, for example, higher rates of distant metastasis, a higher proportion of iodine resistant tumors, and higher mortality than other DTCs [9-11,24]. However, this has been challenged in several studies, which reported that HCC had a similar outcome as follicular thyroid carcinoma [12-14,16]. In the latest population-level analysis that included 3,111 patients with HCC, the overall survival rate was 82.1%, and the disease-specific survival rate was 94.1% [9]. A similar survival outcome was seen in a recently reported single-center data of 239 patients. The 5-year overall survival rates and disease-specific survival rates were 89.4% and 94.6%, respectively [25]. In our study, the overall survival rate was 91.7%, and none of the patients died of thyroid cancer during a median follow-up of 8.5 years. Furthermore, the proportion of patients with persistent/recurrent disease was 8.2%, which was lower than reported in another study (12.1%) with a similar follow-up period [25]. The favorable prognosis observed in our study compared to that in the study by Oluic et al. [25] study, might be due to a low proportion of widely invasive tumors (5% vs. 33%) and larger size tumors (>4 cm, 26% vs. 54%). Furthermore, these results may be related to the early diagnosis and treatment of cancer in this study. Given the rarity of this condition, more studies with larger sample sizes are necessary to validate the findings.
Distant metastasis was found in 4.7% to 11.0% of the patients with HCC in large studies conducted in the United States in recent times [9,11,26]. Previously, Kim et al. [15] reported that distant metastasis was found in 2.5% of the patients with HCC in a study that included some patients from the current study. Similarly, the frequency of synchronous distant metastasis was 3.1% in this study. The reason for the relatively low incidence of distant metastasis in South Korea is unclear. We found that distant metastasis was more frequent in patients with higher age, gross ETE, and widely invasive cancer, which is consistent with previous reports [16].
In this study, we found that higher age, gross ETE, and widely invasive cancer were associated with poor DFS, although only gross ETE and widely invasive cancer were independent risk factors in multivariate analysis. The prognosis of HCC differs between minimally and widely invasive types. Chindris et al. [26] reported that neither recurrence nor disease-specific mortality occurred in 39 patients with minimally invasive HCC, while 62.7% of patients with widely invasive disease had a clinical recurrence or death. Furthermore, widely invasive type, male sex, older age, and higher TNM stage were also thought to be prognostic factors in patients with HCC; however, these studies mainly focused on survival [9,11,26]. Age was a wellknown prognostic factor associated with survival, but not with recurrence.
One of the strengths of this study is that we evaluated the US features of HCC, which are not well known. Due to the rarity of HCC, the US findings have been reported only in a few studies or were analyzed along with those of Hürthle cell adenoma [18]. In the current study, we found that HCC usually showed a solid hypoechoic appearance on US, and on the preoperative cytopathology, FN or suspicious FN was most commonly observed, accounting for 65.2% of the cases according to the Bethesda category. The diagnosis of HCC is challenging because the results of the FNA of HCC are usually non-conclusive for malignancy. The risk of malignancy is only 15% to 30% when HCN is conclusively detected on cytopathology [21,27-29]. In a previous cohort study in South Korea, only 67% of the patients diagnosed with HCC after surgery showed malignancy on preoperative FNA. Therefore, diagnostic thyroid surgery is still necessary based on the tumor characteristics, including US features [16,17,30,31]. This study further strengthens this concept because only 24.7% of HCCs were classified as high-suspicion nodules on the preoperative US, and 21.8% did not show malignancy on preoperative cytopathology in patients who were confirmed to have HCC after surgery.
There were some limitations in this study. First, it was a retrospective study; hence, a potential selection bias might exist. Second, we could not evaluate the clinical factors associated with disease-specific mortality since no patient died of HCC. Third, there might be some differences in the follow-up strategies between the five different centers. However, this is the first multicenter cohort study to evaluate the prognosis of HCC in South Korea.
In summary, to the best of our knowledge, the current study is the largest analysis of patients with HCC in South Korea. The diagnosis of HCC based on US and cytopathology is still challenging. The long-term prognosis of HCC is relatively favorable, with the persistence/recurrence rate of 8.2% during a median follow-up of 8.5 years. Gross ETE and widely invasive cancer were independent risk factors associated with poor DFS.
Supplementary Information
Supplemental Table S1.
Baseline Characteristics of Patients According to the Presence of Distant Metastasis and Persistent/Recurrent Disease
Supplemental Table S2.
Clinical and Ultrasonographic Features of According to the Risk of Persistent/Recurrent Disease
REFERENCES
1. Hundahl SA, Fleming ID, Fremgen AM, Menck HR. A National Cancer Data Base report on 53,856 cases of thyroid carcinoma treated in the U.S., 1985-1995 [see commetns]. Cancer. 1998; 83:2638–48.

2. Hundahl SA, Cady B, Cunningham MP, Mazzaferri E, McKee RF, Rosai J, et al. Initial results from a prospective cohort study of 5583 cases of thyroid carcinoma treated in the United States during 1996. U.S. and German Thyroid Cancer Study Group: an American College of Surgeons Commission on Cancer Patient Care Evaluation study. Cancer. 2000; 89:202–17.

3. Mete O, Asa SL. Oncocytes, oxyphils, Hurthle, and Askanazy cells: morphological and molecular features of oncocytic thyroid nodules. Endocr Pathol. 2010; 21:16–24.

4. Montone KT, Baloch ZW, LiVolsi VA. The thyroid Hurthle (oncocytic) cell and its associated pathologic conditions: a surgical pathology and cytopathology review. Arch Pathol Lab Med. 2008; 132:1241–50.
5. Kure S, Ohashi R. Thyroid Hurthle cell carcinoma: clinical, pathological, and molecular features. Cancers (Basel). 2020; 13:26.

6. Hedinger C, Williams ED, Sobin LH. The WHO histological classification of thyroid tumors: a commentary on the second edition. Cancer. 1989; 63:908–11.

7. Ganly I, Ricarte Filho J, Eng S, Ghossein R, Morris LG, Liang Y, et al. Genomic dissection of Hurthle cell carcinoma reveals a unique class of thyroid malignancy. J Clin Endocrinol Metab. 2013; 98:E962–72.

8. LIoyd RV, Osamura RY, Kloppel G, Rosai J. WHO classification of tumours of endocrine organs. 10th ed. Lyon: International Agency for Research on Cancer;2017.
9. Goffredo P, Roman SA, Sosa JA. Hurthle cell carcinoma: a population-level analysis of 3311 patients. Cancer. 2013; 119:504–11.
10. Phitayakorn R, McHenry CR. Follicular and Hurthle cell carcinoma of the thyroid gland. Surg Oncol Clin N Am. 2006; 15:603–23.
11. Lopez-Penabad L, Chiu AC, Hoff AO, Schultz P, Gaztambide S, Ordonez NG, et al. Prognostic factors in patients with Hurthle cell neoplasms of the thyroid. Cancer. 2003; 97:1186–94.

12. Nagar S, Aschebrook-Kilfoy B, Kaplan EL, Angelos P, Grogan RH. Hurthle cell carcinoma: an update on survival over the last 35 years. Surgery. 2013; 154:1263–71.

13. Khafif A, Khafif RA, Attie JN. Hurthle cell carcinoma: a malignancy of low-grade potential. Head Neck. 1999; 21:506–11.

14. Bhattacharyya N. Survival and prognosis in Hurthle cell carcinoma of the thyroid gland. Arch Otolaryngol Head Neck Surg. 2003; 129:207–10.

15. Kim WG, Yim JH, Kim EY, Kim TY, Gong G, Yoon JH, et al. Preoperative cytological diagnosis of follicular thyroid carcinoma and Huthle cell carcinoma. J Korean Thyroid Assoc. 2010; 3:149–54.
16. Kim WG, Kim TY, Kim TH, Jang HW, Jo YS, Park YJ, et al. Follicular and Hurthle cell carcinoma of the thyroid in iodine-sufficient area: retrospective analysis of Korean multicenter data. Korean J Intern Med. 2014; 29:325–33.

17. Kim TH, Lim JA, Ahn HY, Lee EK, Min HS, Kim KW, et al. Tumor size and age predict the risk of malignancy in Hurthle cell neoplasm of the thyroid and can therefore guide the extent of initial thyroid surgery. Thyroid. 2010; 20:1229–34.

18. Santana NO, Freitas RM, Marcos VN, Chammas MC, Camargo RY, Schmerling CK, et al. Diagnostic performance of thyroid ultrasound in Hurthle cell carcinomas. Arch Endocrinol Metab. 2019; 63:300–5.
19. Shin JH, Baek JH, Chung J, Ha EJ, Kim JH, Lee YH, et al. Ultrasonography diagnosis and imaging-based management of thyroid nodules: revised Korean Society of Thyroid Radiology consensus statement and recommendations. Korean J Radiol. 2016; 17:370–95.

20. Cibas ES, Ali SZ. The 2017 Bethesda system for reporting thyroid cytopathology. Thyroid. 2017; 27:1341–6.

21. Haugen BR, Alexander EK, Bible KC, Doherty GM, Mandel SJ, Nikiforov YE, et al. 2015 American Thyroid Association management guidelines for adult patients with thyroid nodules and differentiated thyroid cancer: the American Thyroid Association Guidelines Task Force on thyroid nodules and differentiated thyroid cancer. Thyroid. 2016; 26:1–133.

22. American Thyroid Association (ATA) Guidelines Taskforce on Thyroid Nodules and Differentiated Thyroid Cancer, Cooper DS, Doherty GM, Haugen BR, Kloos RT, Lee SL, et al. Revised American Thyroid Association management guidelines for patients with thyroid nodules and differentiated thyroid cancer. Thyroid. 2009; 19:1167–214.

23. Jin M, Kim ES, Kim BH, Kim HK, Yi HS, Jeon MJ, et al. Clinical implication of World Health Organization classification in patients with follicular thyroid carcinoma in South Korea: a multicenter cohort study. Endocrinol Metab (Seoul). 2020; 35:618–27.

24. Mills SC, Haq M, Smellie WJ, Harmer C. Hurthle cell carcinoma of the thyroid: retrospective review of 62 patients treated at the Royal Marsden Hospital between 1946 and 2003. Eur J Surg Oncol. 2009; 35:230–4.
25. Oluic B, Paunovic I, Loncar Z, Djukic V, Diklic A, Jovanovic M, et al. Survival and prognostic factors for survival, cancer specific survival and disease free interval in 239 patients with Hurthle cell carcinoma: a single center experience. BMC Cancer. 2017; 17:371.

26. Chindris AM, Casler JD, Bernet VJ, Rivera M, Thomas C, Kachergus JM, et al. Clinical and molecular features of Hurthle cell carcinoma of the thyroid. J Clin Endocrinol Metab. 2015; 100:55–62.

27. Baloch ZW, LiVolsi VA, Asa SL, Rosai J, Merino MJ, Randolph G, et al. Diagnostic terminology and morphologic criteria for cytologic diagnosis of thyroid lesions: a synopsis of the National Cancer Institute Thyroid Fine-Needle Aspiration State of the Science Conference. Diagn Cytopathol. 2008; 36:425–37.

28. Sorrenti S, Trimboli P, Catania A, Ulisse S, De Antoni E, D’Armiento M. Comparison of malignancy rate in thyroid nodules with cytology of indeterminate follicular or indeterminate Hurthle cell neoplasm. Thyroid. 2009; 19:355–60.

29. Pu RT, Yang J, Wasserman PG, Bhuiya T, Griffith KA, Michael CW. Does Hurthle cell lesion/neoplasm predict malignancy more than follicular lesion/neoplasm on thyroid fine-needle aspiration? Diagn Cytopathol. 2006; 34:330–4.

30. Thompson NW, Dunn EL, Batsakis JG, Nishiyama RH. Hurthle cell lesions of the thyroid gland. Surg Gynecol Obstet. 1974; 139:555–60.
31. Yutan E, Clark OH. Hurthle cell carcinoma. Curr Treat Options Oncol. 2001; 2:331–5.
Fig. 1.
(A) Ultrasonographic findings based on the Korean-Thyroid Imaging Reporting and Data System in patients with Hürthle cell carcinoma. (B) Preoperative fine needle aspiration or core needle biopsy based on Bethesda category in patients with Hürthle cell carcinoma. (C) Proportion of Bethesda category according to ultrasonographic feature. AUS, atypia of undetermined significant; FN, follicular neoplasm.
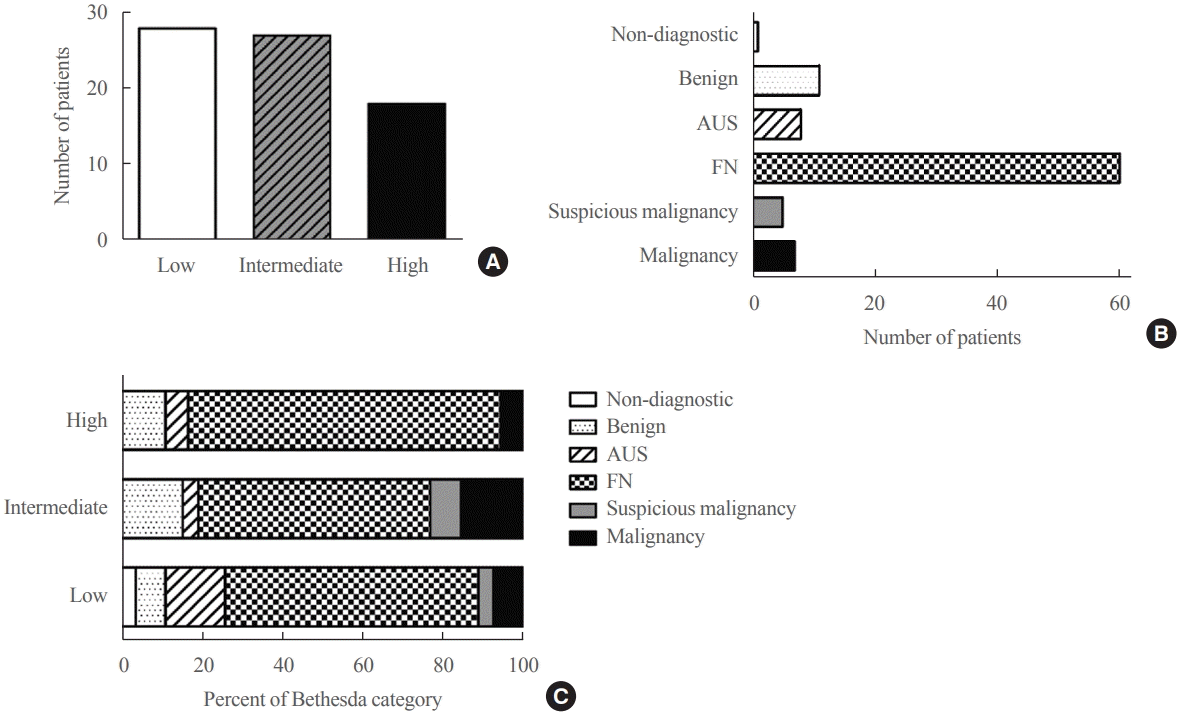
Table 1.
Baseline Characteristics of Patients with Hürthle Cell Carcinoma
Table 2.
Ultrasonographic Features of Hürthle Cell Carcinoma
| Characteristic | Value |
|---|---|
| Number of patients | 73 |
| Composition | |
| Solid | 62 (84.9) |
| Predominantly solid | 11 (15.1) |
| Echogenicity | |
| Markedly hypoechoic | 9 (12.3) |
| Hypoechoic | 40 (54.8) |
| Isoechoic | 24 (32.9) |
| Non-parallel | 10 (13.7) |
| Margin | |
| Smooth | 68 (93.2) |
| Spiculated | 3 (4.1) |
| Ill-defined | 2 (2.7) |
| Calcification | |
| Microcalcification | 7 (9.6) |
| Macrocalcification | 9 (12.3) |
| K-TIRADSa | |
| Low suspicion | 28 (38.4) |
| Intermediate suspicion | 27 (37.0) |
| High suspicion | 18 (24.7) |
| FNA/CNB | |
| Nondiagnostic | 1 (1.1) |
| Benign | 11 (12.0) |
| AUS/FLUS | 8 (8.7) |
| FN or suspicious for FN | 60 (65.2) |
| Suspicious for malignancy | 5 (5.4) |
| Malignancy | 7 (7.6) |
Table 3.
Clinicopathological Features Associated with Disease-Free Survival in Patients with Hürthle Cell Carcinoma




 PDF
PDF Citation
Citation Print
Print



 XML Download
XML Download