Abstract
The impacts of coronavirus disease 2019 (COVID-19) have been globally paradigm shifting in all aspects. Surgeons have experienced unprecedented changes regarding operation schedules, preparations before surgery, and the precautions needed both during and after surgery. Many medical centers simultaneously reported a decrease in their numbers of surgeries, whether they were elective or emergent, or for cancerous or benign resections. However, accumulated surgical outcomes from the last 2 years of experience presented postoperative morbidity and mortality data that were comparable to the pre-pandemic era, whether in elective or urgent settings. Although COVID-19 showed a significant association with postoperative morbidity and mortality, the majority of noninfected patients could be treated successfully with stringent mitigation protocols. Initially recommended to be avoided at the start of the pandemic, minimally invasive surgery seems to be safe and feasible according to reported surgical outcomes. Numerous sets of guidelines have now been produced from medical societies and adhering to the basic precautions has been found to be practicable. It is crucial that health care systems and surgical staff remain vigilant and attentive to the ever-changing situation in this pandemic in order to provide optimal medical support to their patients.
The emergence in December 2019 of a cluster of patients with pneumonia was identified to have been caused by 2019-nCoV, a novel coronavirus [1], which was later classified as severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) [2]. This coronavirus (now referred to globally as coronavirus disease 2019 [COVID-19]) was subsequently announced to be the cause of a global pandemic by the World Health Organization (WHO) on March 11, 2020 [3]. In the 2 years since then, over 452 million cases of COVID-19 have been documented accounting for more than 6 million deaths. Although over 60% of the world’s population has now received a COVID-19 vaccine, around 1 million new cases and 7,000 deaths are still being recorded each day due to this virus. Moreover, the recent dramatic increase of daily COVID-19 patients in the Republic of Korea accounts for almost 30% of the daily new cases being reported worldwide (Fig. 1) [4].
The impacts of COVID-19 have been globally paradigm shifting in all aspects of people’s daily lives. From a clinical perspective, surgeons have also experienced unprecedented changes due to this pandemic regarding operation schedules, preparations before surgery, and the precautions needed both during and after surgery. Prior experiences with Middle East respiratory syndrome and SARS-CoV have helped with this to some degree, but the magnitude of the spread of COVID-19 has been incomparable in this regard [5]. A global expert response study was conducted just after the declaration of the pandemic in an effort to estimate the worldwide cancellation of surgeries that would occur. This study included all 193 United Nations member countries except Liechtenstein, North Korea, and Somalia. The number of canceled surgeries worldwide was estimated to have ranged from 19 to 43 million in the peak 12 weeks of the COVID-19 outbreak, of which the duration was wildly underestimated. More than 80% of elective surgeries and 30% of cancer surgeries were anticipated to have been canceled [6].
There were obvious concerns that necessary surgeries would not be provided in time for many patients in need and that this would have a detrimental influence on short-term survival outcomes. Cases of malignant disease were, unsurprisingly, of particular concern due to not only the delayed surgery but also the postponements of surveillance or regular check-ups. Surgical societies across the world have produced designated guidelines and recommendations to cope with the impact of the pandemic. Anticipated shortages of intensive care beds and medical personnel have been noted as a potentially critical issue for patients in need of emergent surgeries for acute abdominal issues and for oncologic safety. Guidelines announced at the start of the pandemic were largely focused on treating emergent patients and updates were published to reflect the accumulated clinical data.
This review aimed to condense the vast clinical data that has been shared globally in order to accurately summarize the current surgical practices in the COVID-19 era, the influence that the pandemic has had on surgical outcomes, and the safety criteria issued by surgical societies to assist their members with the impacts of this pandemic.
A literature search for eligible studies was undertaken in March 2022 using PubMed databases. Search keywords included “COVID” and “surgery” or “surgical.” Initial searches revealed more than 1.6 million published reports in both 2020 and 2021. There were a further 440,000 results in the first 3 months of 2022. From these voluminous search results, review articles reported in major journals related to general surgery were preferentially selected for a more detailed perusal. Abstracts were screened to identify multinational multicenter-based studies to be evaluated as a priority. Grey literature announced by health organizations based on national or global health authorities were identified from references in the included published research papers. All levels of evidence were included. All studies presenting clinical outcomes after surgery during the pandemic period were thoroughly reviewed.
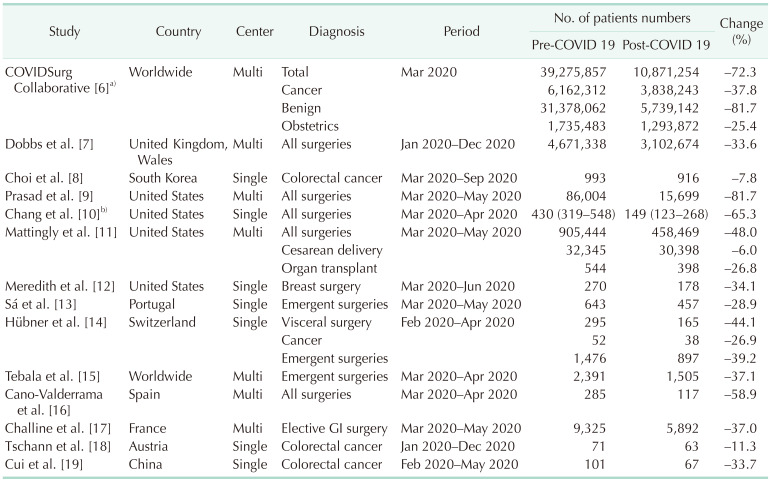
Throughout the course of the pandemic, many medical centers simultaneously reported a decrease in their numbers of surgeries, whether they were elective or emergent, or for cancerous or benign resections. The initial prediction made by the COVIDSurg Collaborative group was an 80% decrease in benign and a 30% decrease in malignant cancer surgeries (Table 1) [678910111213141516171819]. A multicenter-based report from England and Wales reported a 33.6% decrease in all types of surgeries in 2020 compared to non-pandemic periods (4,671,338 to 3,102,674) [7]. Similarly, elective surgery cases in the United States (US) showed a dramatic decrease (34.1% to 81.7%) in several reports, including 2 multicenter studies in the period from March to May 2020 compared to the same months in 2019 [9101112]. In addition to elective surgeries, emergent operations have also been reported to decrease by around 30% worldwide [131415]. Cancer surgeries and obstetric operations have been less affected with centers reporting only a 7.8% reduction in colorectal cancer resections [8] and a 6% drop in cesarean deliveries [11]. Notably, these decreases in the US recovered to nearly 2019 levels after healthcare systems adapted to the pandemic, even during the peak burden of the disease [911]. A national observational study conducted in England predicted that by the end of 2021, 2.4 million surgeries would be overdue [7]. A nearly 5 million surgical case backlog has been estimated in the US alone, and it is clear that dealing with this will be an arduous task [20].
Guidelines from various surgical societies issued in March 2020 mostly recommended postponing elective surgeries indefinitely and focusing on emergency operations. Reducing elective operations had benefits in releasing general ward and intensive care unit beds to treat COVID-19 patients. Also, there was no information on outcomes after surgery during the pandemic at that time [21]. At present, however, centers around the world have been reporting the clinical outcomes of elective operations during the pandemic period.
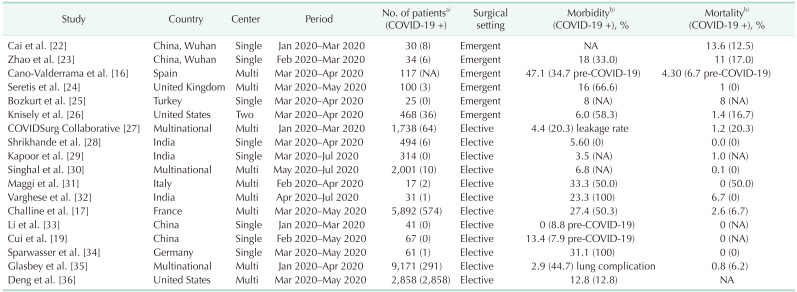
Twelve studies were reviewed for elective surgery outcomes during the COVID-19 pandemic (Table 2) [161719222324252627282930313233343536]. All of these reports presented postoperative morbidity and mortality data that were comparable to the pre-pandemic era. The average leakage rate after colorectal cancer surgeries was 4.9% and the mortality rate was 1.8%. Postoperative COVID-19 infection was significantly associated with mortality (adjusted odds ratio, 16.90; P < 0.001), which required surgeons to undertake stringent mitigation measures [27]. Furthermore, the leakage rate presented in this study was comparable to those of around 10% reported in the pre-pandemic era [3738]. In addition, a further study reported a 10-fold increase in mortality among symptomatic COVID-19 patients but only a 2-fold increase in asymptomatic patients after gastrointestinal surgery. Systematic screening for the virus has therefore been recommended before conducting elective surgeries [17].
A recent retrospective study in the US has presented the results of major elective surgeries in patients infected with COVID-19. Surgery within 0 to 4 weeks of a positive COVID-19 test was found to be significantly associated with pulmonary complications, while patients who underwent surgery more than 8 weeks after a COVID-19 infection did not show any increased morbidity [36]. Other studies have presented similar results, thus recommending that COVID-19 positive patients not undergo any surgery until at least 7 to 8 weeks have passed [3940].
In spite of the higher risk of postoperative morbidity (especially pulmonary complications) in COVID-19 patients, and the possibility of spreading the virus during hospitalization and surgery, there are always going to be situations where an immediate surgical intervention has to be performed to save the life of the patient. From the start of the pandemic in March 2020, many studies have repeatedly insisted that surgeons should not hesitate to perform urgent operations when necessary. Six studies were reviewed regarding surgical outcomes after urgent operations during the COVID-19 era (Table 2) [162223242526]. These reports included positive COVID-19 cases. Although COVID-19 again showed a significant association with postoperative morbidity and mortality [26], the majority of noninfected patients could be treated successfully with stringent mitigation protocols in place, and the outcomes, including adverse events, were comparable to those found in the pre-pandemic period [41]. The postoperative COVID-19 infection rate was reported to be around 3% [24].
Tumor progression can occur in as little as 4 to 8 weeks in certain cases and delaying surgery in colorectal cancer by more than 30 to 40 days is reported to be associated with poorer overall survival in several studies [424344]. A prior systemic review and meta-analysis had recommended that colorectal cancer surgery should not be postponed for longer than 4 weeks [45]. The tumor volume doubling time of a primary lesion is dependent on the type of cancer and its organ of origin but delaying a resection procedure by more than 30 days is generally reported to be unfavorable [464748].
As noted in an earlier section, the backlog of surgeries due to COVID-19 in England now represents more than 6 months of pre-pandemic surgical procedures [7]. It has also been predicted that it will require up to 3 months to clear the current backlog of overdue surgeries in the US alone [20]. Hence, restoring surgical capacities to pre-pandemic levels is important and revisions to current clinical practices should be considered to achieve this, if feasible. The American College of Surgeons guidelines for elective cases recommend considering neoadjuvant chemotherapy for 2 to 3 months in colon cancer patients in the COVID-19 era [49]. Also, the Society of Surgical Oncology recommends neoadjuvant therapy for resectable pancreatic cancer and selected gastric cancer cases as a temporary measure during the pandemic [50]. Although resection of primary cancer is the treatment of choice for most gastrointestinal malignancies, advances in adjuvant treatments have provided clinicians with alternative measures to cope with the current circumstances [515253].
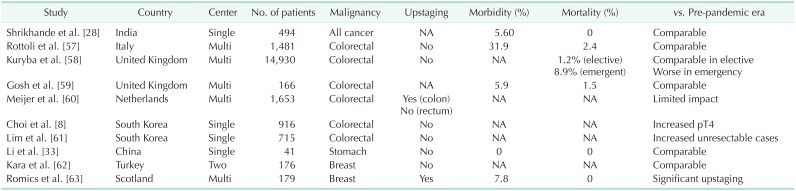
Some reports have indicated that cancer patients are more vulnerable to the severe complications associated with COVID-19 [5455]. Despite this, the continuance of cancer treatment is recommended by the Korean Cancer Association [56]. Clinicians must treat cancer patients in accordance with their individual characteristics, the involved organ(s), the tumor stage, and the available adjuvant treatments. Although some studies have indicated an increase in T4 stage cancer during the pandemic, most have reported no significant upstaging in terms of the TNM classification (Table 3) [8283357585960616263]. A hasty or obstinate selection of surgical resection is thus unnecessary in positive COVID-19 cases if other treatment modalities are available. Furthermore, the use of telehealth or telemedicine applications, in which clinicians assess and manage patients without direct contact, has also emerged and been adopted rapidly as a countermeasure [51646566].
Virus transmission during the pandemic has been one of the prime concerns for surgeons. Airborne spread may occur not only during intubation and extubation but also during the procedures when fumes are created by surgical devices [67]. Early in the COVID-19 era, a study proposed that although there was insufficient evidence to prove that minimally invasive surgery (MIS) was safe in terms of transmission, this method could be performed while adhering to necessary precautions. Recommendations from the United Kingdom and Ireland Intercollegiate Board, however, were that MIS was generally not to be used during the pandemic and that laparoscopy should be considered only in selected individual cases [68].
After 2 years of surgical experiences during the COVID19 pandemic, a number of studies have reported that MIS is safe and feasible. A study in England revealed no difference between MIS and open surgery regarding the transmission rate to 14 staff members during 73 operations [69]. A Japanese group quantified the particulate matter counts in surgical smoke from both laparoscopic and open surgery in colorectal disease. The results showed lower exposure to surgical smoke during laparoscopic surgery compared to open surgery [70]. The Society of American Gastrointestinal and Endoscopic Surgeons (SAGES) summarized studies on MIS during the pandemic era and reported its results recently. A case report did not detect viral particles in the surgical fume directly taken from the trocar [71], and a case series detected SARS-CoV-2 in the surgical plume but no medical staff member was infected due to the exposure [72]. SAGES summarized MIS to be safe and concluded that either MIS or an open approach can be performed in COVID-19 patients [73].
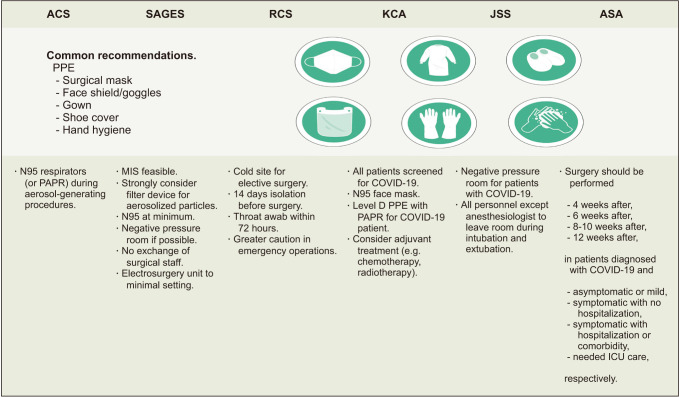
Numerous sets of guidelines have now been produced from medical societies around the world since the beginning of the COVID19 pandemic. There are slight distinctions between them as they aim to optimize procedures in accordance with the requirements of each nation’s population (Fig. 2) [6874757677]. Overall, the backbone of most sets of guidelines is based on WHO and Centers for Disease Control and Prevention recommendations, which are also supported by evidence from various reports. The interim guidance announced by WHO in June 2020 recommended molecular assays of upper respiratory specimens from any patient showing signs or symptoms of COVID-19. This can be modified depending on local testing capacity and testing should also be conducted on all patients before a surgical procedure regardless of the risk assessment for COVID-19. Staff participating in surgical procedures for a COVID-19 patient should use sterile medical mask, face shield or goggles, gloves, and gown. An N95 mask should be used when aerosol-generating procedures are being conducted. A negative pressure room would be ideal in these instances if available [78]. Dedicated operating theatres for COVID-19 patients are also recommended but can be used for negative patients after terminal cleaning. The number of surgical staff in the theatre should also be limited to essential personnel only [79]. Patients should be isolated for at least 14 days if they have had contact with COVID-19 patients prior to surgery [80]. The Korean Cancer Association suggests that all patients be screened for COVID-19 prior to surgery and that personal protective equipment (PPE) for surgical staff should include double gloves, N95 masks, face shields or goggles, surgical caps, surgical gowns, and surgical shoe covers for all procedures. Level D PPE powered using an air-purifying respirator (powered air-purifying respirator [PAPR]) is also recommended for performing surgery on COVID-19 patients [59]. Recommendations from selected international societies are summarized in Fig. 2.
The challenges facing surgical teams during the COVID-19 pandemic remain ongoing. Guidelines were mostly enacted shortly after the pandemic was officially announced and are being continually updated in accordance with its progression. However, COVID-19 is showing a peak surge at the time of writing compared to the past 2 years and the existing guidelines are not based on the most severe recent rise in COVID-19 patients. Predictions of the pandemic’s course have now been widely exceeded and it would also be a conjecture to consider that the current situation could not worsen further.
It must be noted, however, that performing essential surgeries for patients in need has proven to be feasible during this health crisis. Adherence to the basic precautions from global health organizations and to any modified recommendations from local surgical organizations has been found to be practicable, as reported by various studies from different regions of the world. It is crucial, that health care systems and surgical staff remain vigilant and attentive to the ever-changing situation in this pandemic in order to provide optimal medical support to their patients.
There were several limitations to this present review of note. Although articles related to surgical safety in the COVID-19 era were searched as thoroughly as possible, it was obviously not possible to review all of the more than 3 million papers published to date and some information could have been overlooked. Also, as previously discussed, the data from the included studies in our present review do not reflect the current situation in the pandemic or the progression of the COVID-19 virus.
References
1. Zhu N, Zhang D, Wang W, Li X, Yang B, Song J, et al. A novel coronavirus from patients with pneumonia in China, 2019. N Engl J Med. 2020; 382:727–733. PMID: 31978945.

2. Coronaviridae Study Group of the International Committee on Taxonomy of Viruses. The species Severe acute respiratory syndrome-related coronavirus: classifying 2019-nCoV and naming it SARS-CoV-2. Nat Microbiol. 2020; 5:536–544. PMID: 32123347.
3. World Health Organization (WHO). WHO Director-General’s opening remarks at the media briefing on COVID-19 - 11 March 2020 [Internet]. Geneva: WHO;2020. cited 2022 Apr 11. Available from: https://www.who.int/director-general/speeches/detail/who-director-general-s-opening-remarks-at-the-media-briefing-on-covid-19---11-march-2020.
4. Johns Hopkins University & Medicine. COVID-19 dashboard by the Center for Systems Science and Engineering (CSSE) at Johns Hopkins University (JHU) [Internet]. Baltimore, MD: Johns Hopkins University & Medicine;c2022. cited 2022 Apr 11. Available from: https://coronavirus.jhu.edu/map.html.
5. Berber E, Sumbria D, Çanakoğlu N. Meta-analysis and comprehensive study of coronavirus outbreaks: SARS, MERS and COVID-19. J Infect Public Health. 2021; 14:1051–1064. PMID: 34174535.

6. COVIDSurg Collaborative. Elective surgery cancellations due to the COVID-19 pandemic: global predictive modelling to inform surgical recovery plans. Br J Surg. 2020; 107:1440–1449. PMID: 32395848.
7. Dobbs TD, Gibson JA, Fowler AJ, Abbott TE, Shahid T, Torabi F, et al. Surgical activity in England and Wales during the COVID-19 pandemic: a nationwide observational cohort study. Br J Anaesth. 2021; 127:196–204. PMID: 34148732.

8. Choi JY, Park IJ, Lee HG, Cho E, Kim YI, Kim CW, et al. Impact of the COVID-19 pandemic on surgical treatment patterns for colorectal cancer in a tertiary medical facility in Korea. Cancers (Basel). 2021; 13:2221. PMID: 34066390.

9. Prasad NK, Englum BR, Turner DJ, Lake R, Siddiqui T, Mayorga-Carlin M, et al. A nation-wide review of elective surgery and COVID-surge capacity. J Surg Res. 2021; 267:211–216. PMID: 34157490.

10. Chang EI, Liu JJ. Flattening the curve in oncologic surgery: impact of COVID-19 on surgery at tertiary care cancer center. J Surg Oncol. 2020; 06. 02. DOI: 10.1002/jso.26056. [Epub].

11. Mattingly AS, Rose L, Eddington HS, Trickey AW, Cullen MR, Morris AM, et al. Trends in US surgical procedures and health care system response to policies curtailing elective surgical operations during the COVID-19 pandemic. JAMA Netw Open. 2021; 4:e2138038. PMID: 34878546.

12. Meredith JW, High KP, Freischlag JA. Preserving elective surgeries in the COVID-19 pandemic and the future. JAMA. 2020; 324:1725–1726. PMID: 33031523.

13. Sá AF, Lourenço SF, Teixeira RD, Barros F, Costa A, Lemos P. Urgent/emergency surgery during COVID-19 state of emergency in Portugal: a retrospective and observational study. Braz J Anesthesiol. 2021; 71:123–128. PMID: 33623174.

14. Hübner M, Zingg T, Martin D, Eckert P, Demartines N. Surgery for non-COVID-19 patients during the pandemic. PLoS One. 2020; 15:e0241331. PMID: 33095834.

15. Tebala GD, Milani MS, Bignell M, Bond-Smith G, Lewis C, Cirocchi R, et al. Emergency surgery admissions and the COVID-19 pandemic: did the first wave really change our practice?: results of an ACOI/WSES international retrospective cohort audit on 6263 patients. World J Emerg Surg. 2022; 17:8. PMID: 35090519.
16. Cano-Valderrama O, Morales X, Ferrigni CJ, Martín-Antona E, Turrado V, García A, et al. Acute care surgery during the COVID-19 pandemic in Spain: changes in volume, causes and complications: a multicentre retrospective cohort study. Int J Surg. 2020; 80:157–161. PMID: 32679205.

17. Challine A, Dousset B, de’Angelis N, Lefèvre JH, Parc Y, Katsahian S, et al. Impact of coronavirus disease 2019 (COVID-19) lockdown on in-hospital mortality and surgical activity in elective digestive resections: a nationwide cohort analysis. Surgery. 2021; 170:1644–1649. PMID: 33597086.

18. Tschann P, Girotti PN, Lechner D, Adler S, Feurstein B, Szeverinski P, et al. How does the COVID-19 pandemic influence surgical case load and histological outcome for colorectal cancer?: a single-centre experience. J Gastrointest Surg. 2021; 25:2957–2960. PMID: 33852126.

19. Cui J, Li Z, An Q, Xiao G. Impact of the COVID-19 pandemic on elective surgery for colorectal cancer. J Gastrointest Cancer. 2021; 03. 17. DOI: 10.1007/s12029-021-00621-1. [Epub].

20. Fu SJ, George EL, Maggio PM, Hawn M, Nazerali R. The consequences of delaying elective surgery: surgical perspective. Ann Surg. 2020; 272:e79–e80. PMID: 32675504.

21. COVIDSurg Collaborative. Global guidance for surgical care during the COVID-19 pandemic. Br J Surg. 2020; 107:1097–1103. PMID: 32293715.
22. Cai M, Wang G, Zhang L, Gao J, Xia Z, Zhang P, et al. Performing abdominal surgery during the COVID-19 epidemic in Wuhan, China: a single-centred, retrospective, observational study. Br J Surg. 2020; 107:e183–e185. PMID: 32339259.

23. Zhao N, Wu L, Cheng Y, Zheng H, Hu P, Hu C, et al. The effect of emergency surgery on acute abdomen patients with COVID-19 pneumonia: a retrospective observational study. Aging (Albany NY). 2020; 12:15771–15783. PMID: 32805726.

24. Seretis C, Archer L, Lalou L, Yahia S, Katz C, Parwaiz I, et al. Minimal impact of COVID-19 outbreak on the postoperative morbidity and mortality following emergency general surgery procedures: results from a 3-month observational period. Med Glas (Zenica). 2020; 17:275–278. PMID: 32662615.
25. Bozkurt H, Gür HÜ, Akıncı M, Aslan H, Karakullukçu Ç, Yıldırım D. Evaluation of patients undergoing emergency surgery in a COVID-19 pandemic hospital: a cross-sectional study. Sao Paulo Med J. 2020; 138:305–309. PMID: 32638937.

26. Knisely A, Zhou ZN, Wu J, Huang Y, Holcomb K, Melamed A, et al. Perioperative morbidity and mortality of patients with COVID-19 who undergo urgent and emergent surgical procedures. Ann Surg. 2021; 273:34–40. PMID: 33074900.

27. COVIDSurg Collaborative. Outcomes from elective colorectal cancer surgery during the SARS-CoV-2 pandemic. Colorectal Dis. 2020; 11. 15. DOI: 10.1111/codi.15431. [Epub].
28. Shrikhande SV, Pai PS, Bhandare MS, Bakshi G, Chaukar DA, Chaturvedi P, et al. Outcomes of elective major cancer surgery during COVID 19 at Tata Memorial Centre: implications for cancer care policy. Ann Surg. 2020; 272:e249–e252. PMID: 32520743.
29. Kapoor D, Perwaiz A, Singh A, Chaudhary A. Elective gastrointestinal surgery in COVID times. Indian J Surg. 2020; 10. 22. DOI: 10.1007/s12262-020-02642-9. [Epub].
30. Singhal R, Tahrani AA, Ludwig C, Mahawar K. GENEVA collaborators. Global 30-day outcomes after bariatric surgery during the COVID-19 pandemic (GENEVA): an international cohort study. Lancet Diabetes Endocrinol. 2021; 9:7–9. PMID: 33253631.
31. Maggi U, De Carlis L, Yiu D, Colledan M, Regalia E, Rossi G, et al. The impact of the COVID-19 outbreak on liver transplantation programs in Northern Italy. Am J Transplant. 2020; 20:1840–1848. PMID: 32330351.

32. Varghese J, Malleeswaran S, Patcha RV, Appusamy E, Karnan P, Kapoor D, et al. A multicentric experience on living donor liver transplantation in coronavirus disease 2019 hotspots in India. Liver Transpl. 2021; 27:1334–1338. PMID: 33253477.

33. Li YX, He CZ, Liu YC, Zhao PY, Xu XL, Wang YF, et al. The impact of COVID-19 on gastric cancer surgery: a single-center retrospective study. BMC Surg. 2020; 20:222. PMID: 33008379.

34. Sparwasser P, Brandt MP, Haack M, Dotzauer R, Boehm K, Gheith MK, et al. Robotic surgery can be safely performed for patients and healthcare workers during COVID-19 pandemic. Int J Med Robot. 2021; 17:e2291. PMID: 34050598.

35. Glasbey JC, Nepogodiev D, Simoes JF, Omar O, Li E, Venn ML, et al. Elective cancer surgery in COVID-19-free surgical pathways dur ing the SARS-CoV-2 pandemic: an international, multicenter, comparative cohort study. J Clin Oncol. 2021; 39:66–78. PMID: 33021869.
36. Deng JZ, Chan JS, Potter AL, Chen YW, Sandhu HS, Panda N, et al. The risk of postoperative complications after major elective surgery in active or resolved COVID-19 in the United States. Ann Surg. 2022; 275:242–246. PMID: 34793348.

37. Oh CK, Huh JW, Lee YJ, Choi MS, Pyo DH, Lee SC, et al. Long-term oncologic outcome of postoperative complications after colorectal cancer surgery. Ann Coloproctol. 2020; 36:273–280. PMID: 32054256.

38. Chaouch MA, Kellil T, Jeddi C, Saidani A, Chebbi F, Zouari K. How to prevent anastomotic leak in colorectal surgery?: a systematic review. Ann Coloproctol. 2020; 36:213–222. PMID: 32919437.

39. GlobalSurg Collaborative. COVIDSurg Collaborative. Timing of surgery following SARS-CoV-2 infection: an international prospective cohort study. Anaesthesia. 2021; 76:748–758. PMID: 33690889.
40. Kovoor JG, Scott NA, Tivey DR, Babidge WJ, Scott DA, Beavis VS, et al. Proposed delay for safe surgery after COVID-19. ANZ J Surg. 2021; 91:495–506. PMID: 33656269.

41. Tebala GD, Mingoli A, Natili A, Khan AQ, Brachini G. Surgical risk and pathological results of emergency resection in the treatment of acutely obstructing colorectal cancers: a retrospective cohort study. Ann Coloproctol. 2021; 37:21–28. PMID: 32178504.

42. Grass F, Behm KT, Duchalais E, Crippa J, Spears GM, Harmsen WS, et al. Impact of delay to surgery on survival in stage I-III colon cancer. Eur J Surg Oncol. 2020; 46:455–461. PMID: 31806516.

43. Kaltenmeier C, Shen C, Medich DS, Geller DA, Bartlett DL, Tsung A, et al. Time to surgery and colon cancer survival in the United States. Ann Surg. 2021; 274:1025–1031. PMID: 31850985.

44. Shin DW, Cho J, Kim SY, Guallar E, Hwang SS, Cho B, et al. Delay to curative surgery greater than 12 weeks is associated with increased mortality in patients with colorectal and breast cancer but not lung or thyroid cancer. Ann Surg Oncol. 2013; 20:2468–2476. PMID: 23529782.

45. Whittaker TM, Abdelrazek ME, Fitzpatrick AJ, Froud JL, Kelly JR, Williamson JS, et al. Delay to elective colorectal cancer surgery and implications for survival: a systematic review and meta-analysis. Colorectal Dis. 2021; 23:1699–1711. PMID: 33714235.

46. Klein CA. Cancer progression and the invisible phase of metastatic colonization. Nat Rev Cancer. 2020; 20:681–694. PMID: 33024261.

47. Marchegiani G, Andrianello S, Perri G, Secchettin E, Maggino L, Malleo G, et al. Does the surgical waiting list affect pathological and survival outcome in resectable pancreatic ductal adenocarcinoma? HPB (Oxford). 2018; 20:411–417. PMID: 29191689.

48. Sanjeevi S, Ivanics T, Lundell L, Kartalis N, Andrén-Sandberg Å, Blomberg J, et al. Impact of delay between imaging and treatment in patients with potentially curable pancreatic cancer. Br J Surg. 2016; 103:267–275. PMID: 26572509.

49. American College of Surgeons (ACS). COVID-19: elective case triage guidelines for surgical care [Internet]. Chicago, IL: ACS;c1996-2022. cited 2022 Apr 11. Available from: https://www.facs.org/for-medical-professionals/covid-19/clinical-guidance/elective-case/.
50. Society of Surgical Oncology (SSO). COVID-19 resources. Rosemont, IL: SOS;2020. cited 2022 Apr 11. Available from: https://www.surgonc.org/resources/covid-19-resources/.
51. Manlubatan SI, Lopez MP, Onglao MA, Monroy Iii HJ. Modifications to treatment plan of rectal cancer in response to COVID-19 at the Philippine General Hospital. Ann Coloproctol. 2021; 37:225–231. PMID: 34364319.

52. Park SH, Shin JK, Lee WY, Yun SH, Cho YB, Huh JW, et al. Clinical outcomes of neoadjuvant chemotherapy in colorectal cancer patients with synchronous resectable liver metastasis: a propensity score matching analysis. Ann Coloproctol. 2021; 37:244–252. PMID: 34182620.

53. Park EJ. Shifting treatment strategies to prevent early relapse of locally advanced rectal cancer after preoperative chemoradiotherapy. Ann Coloproctol. 2020; 36:357–358. PMID: 33486905.

54. Liang W, Guan W, Chen R, Wang W, Li J, Xu K, et al. Cancer patients in SARS-CoV-2 infection: a nationwide analysis in China. Lancet Oncol. 2020; 21:335–337. PMID: 32066541.

55. Zhang L, Zhu F, Xie L, Wang C, Wang J, Chen R, et al. Clinical characteristics of COVID-19-infected cancer patients: a retrospective case study in three hospitals within Wuhan, China. Ann Oncol. 2020; 31:894–901. PMID: 32224151.

56. Lee JB, Jung M, Kim JH, Kim BH, Kim Y, Kim YS, et al. Guidelines for cancer care during the COVID-19 pandemic in South Korea. Cancer Res Treat. 2021; 53:323–329. PMID: 33721486.

57. Rottoli M, Pellino G, Spinelli A, Flacco ME, Manzoli L, Morino M, et al. Impact of COVID-19 on the oncological outcomes of colorectal cancer surgery in northern Italy in 2019 and 2020: multicentre comparative cohort study. BJS Open. 2022; 6:zrab139. PMID: 35143629.
58. Kuryba A, Boyle JM, Blake HA, Aggarwal A, van der Meulen J, Braun M, et al. Surgical treatment and outcomes of colorectal cancer patients during the COVID-19 pandemic: a national population-based study in England. Ann Surg Open. 2021; 2:e071. PMID: 34240077.

59. Ghosh S, Nevins EJ, Hicks GJ, Carney K, Emmett C, Mills SJ. Colorectal cancer care in the COVID-19 era: outcomes from a‘mixed site’ model. Ann R Coll Surg Engl. 2022; 104:261–268. PMID: 34846184.
60. Meijer J, Elferink MA, van Hoeve JC, Buijsen J, van Erning F, Nagtegaal ID, et al. Impact of the COVID-19 pandemic on colorectal cancer care in the Netherlands: a population-based study. Clin Colorectal Cancer. 2022; 03. 03. DOI: 10.1016/j.clcc.2022.02.005. [Epub].

61. Lim JH, Lee WY, Yun SH, Kim HC, Cho YB, Huh JW, et al. Has the COVID-19 pandemic caused upshifting in colorectal cancer stage? Ann Coloproctol. 2021; 37:253–258. PMID: 34376026.

62. Kara H, Arikan AE, Dulgeroglu O, Tutar B, Tokat F, Uras C. Has the COVID-19 pandemic affected breast cancer stage and surgical volume? Front Surg. 2022; 9:811108. PMID: 35198597.

63. Romics L, Doughty J, Stallard S, Mansell J, Blackhall V, Lannigan A, et al. A prospective cohort study of the safety of breast cancer surgery during COVID-19 pandemic in the West of Scotland. Breast. 2021; 55:1–6. PMID: 33285400.

64. Monaghesh E, Hajizadeh A. The role of telehealth during COVID-19 outbreak: a systematic review based on current evidence. BMC Public Health. 2020; 20:1193. PMID: 32738884.

65. Simcock R, Thomas TV, Estes C, Filippi AR, Katz MA, Pereira IJ, et al. COVID-19: global radiation oncology’s targeted response for pandemic preparedness. Clin Transl Radiat Oncol. 2020; 22:55–68. PMID: 32274425.

66. Kim YJ. The future medical science and colorectal surgeons. Ann Coloproctol. 2017; 33:207–209. PMID: 29354602.

67. Mowbray N, Ansell J, Warren N, Wall P, Torkington J. Is surgical smoke harmful to theater staff?: a systematic review. Surg Endosc. 2013; 27:3100–3107. PMID: 23605191.

68. The Royal College of Surgeons of Edinburgh. Intercollegiate general surgery guidance on COVID-19 update [Internet]. Edinburgh: The Royal College of Surgeons of Edinburgh;2020. cited 2022 Apr 11. Available from: https://www.rcsed.ac.uk/news-public-affairs/news/2020/march/intercollegiate-general-surgery-guidance-on-covid-19-update.
69. Hadjittofi C, Seraj SS, Uddin A, Ali ZJ, Antonas PL, Fisher RJ, et al. Laparoscopic vs open surgery during the COVID-19 pandemic: what are the risks. Ann R Coll Surg Engl. 2021; 103:354–359. PMID: 33682443.

70. Kameyama H, Otani T, Yamazaki T, Iwaya A, Uehara H, Harada R, et al. Comparison of surgical smoke between open surgery and laparoscopic surgery for colorectal disease in the COVID-19 era. Surg Endosc. 2022; 36:1243–1250. PMID: 33616729.

71. Romero-Velez G, Pereira X, Zenilman A, Camacho D. SARS-Cov-2 was not found in the peritoneal fluid of an asymptomatic pat ient undergoing laparoscopic appendectomy. Surg Laparosc Endosc Percutan Tech. 2020; 30:e43–e45. PMID: 32694404.
72. Bogani G, Ditto A, De Cecco L, Lopez S, Guerrisi R, Piccioni F, et al. Transmission of SARS-CoV-2 in surgical smoke during laparoscopy: a prospective, proof-of-concept study. J Minim Invasive Gynecol. 2021; 28:1519–1525. PMID: 33373728.

73. Collings AT, Jeyarajah DR, Hanna NM, Dort J, Tsuda S, Nepal P, et al. SAGES 2022 guidelines regarding the use of laparoscopy in the era of COVID-19. Surg Endosc. 2022; 36:2723–2733. PMID: 35237900.

74. Tan WJ, Foo FJ, Sivarajah SS, Li LH, Koh FH, Chew MH. Safe colorectal surgery in the COVID-19 era: a Singapore experience. Ann Coloproctol. 2020; 36:65–69. PMID: 32429009.

75. American College of Surgeons (ACS). COVID 19: considerations for optimum surgeon protection before, during, and after operation [Internet]. Chicago, IL: ACS;2020. updated 2020 Apr 1. cited 2020 Apr 5. Available from: https://www.facs.org/for-medical-professionals/covid-19/clinical-guidance/surgeon-protection/.
76. Pryor A. SAGES and EAES recommendations regarding surgical response to COVID-19 crisis. Los Angeles, CA: Society of American Gastrointestinal and Endoscopic Surgeons (SAGES);c2022. released 2020 Mar 30. cited 2022 Apr 11. Available from: https://www.sages.org/recommendations-surgical-response-covid-19/.
77. American Society of Anesthesiologist (ASA). ASA and APSF joint statement on elective surgery and anesthesia for patients after COVID-19 infection [Internet]. Schaumburg, IL: ASA;c2022. cited 2022 Apr 11. Available from: https://www.asahq.org/about-asa/newsroom/news-releases/2020/12/asa-and-apsf-joint-statement-on-elective-surgery-and-anesthesia-for-patients-after-covid-19-infection.
78. Mori M, Ikeda N, Taketomi A, Asahi Y, Takesue Y, Orimo T, et al. COVID-19: clinical issues from the Japan Surgical Society. Surg Today. 2020; 50:794–808. PMID: 32651686.

79. World Health Organization (WHO). Infection prevention and control during health care when coronavirus disease (COVID-19) is suspected or confirmed. Interim guidance, 29 June 2020 [Internet]. Geneva: WHO;c2022. cited 2022 Apr 11. Available from: https://www.who.int/publications/i/item/WHO-2019-nCoV-IPC-2021.1.
80. Centers for Disease Control and Prevention (CDC). Duration of isolation and precautions for adults with COVID-19. Atlanta, GA: CDC;2020. cited 2022 Apr 11. Available from: https://stacks.cdc.gov/view/cdc/93433/cdc_93433_DS1.pdf?.
Fig. 1
Coronavirus disease 2019 (COVID-19) trends of recent 2 years. The recent surge of active COVID-19 from January 2022 in South Korea accounts for the global abrupt increase of active COVID-19. Charts have been edited using data provided by COVID-19 Dashboard (https://coronaboard.com/global/).
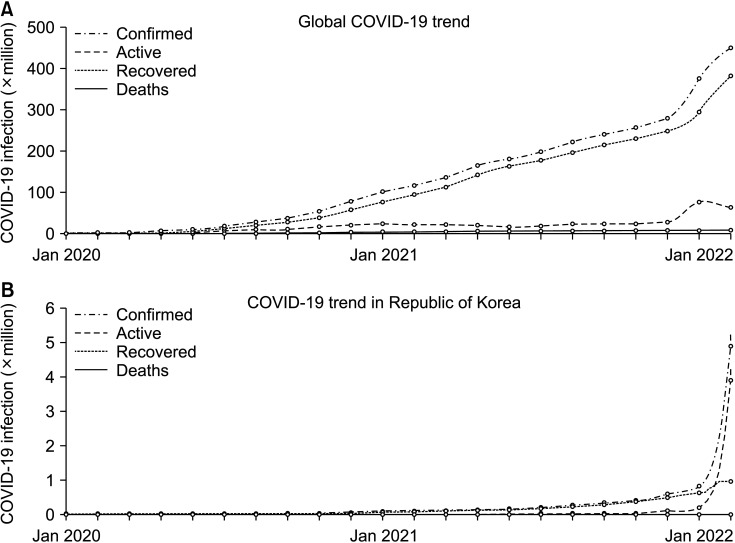
Fig. 2
Recommendations from medical societies on coronavirus disease 2019 (COVID-19) precautions regarding surgery. ACS, American College of Surgeons; SAGES, Society of American Gastrointestinal and Endoscopic Surgeons; RCS, Royal College of Surgeons; KCA, Korean Cancer Association; JSS, Japan Surgical Society; ASA, American Society of Anesthesiologists; PPE, personal protective equipment; PAPR, purifying respirator; ICU, intensive care unit.




 PDF
PDF Citation
Citation Print
Print



 XML Download
XML Download