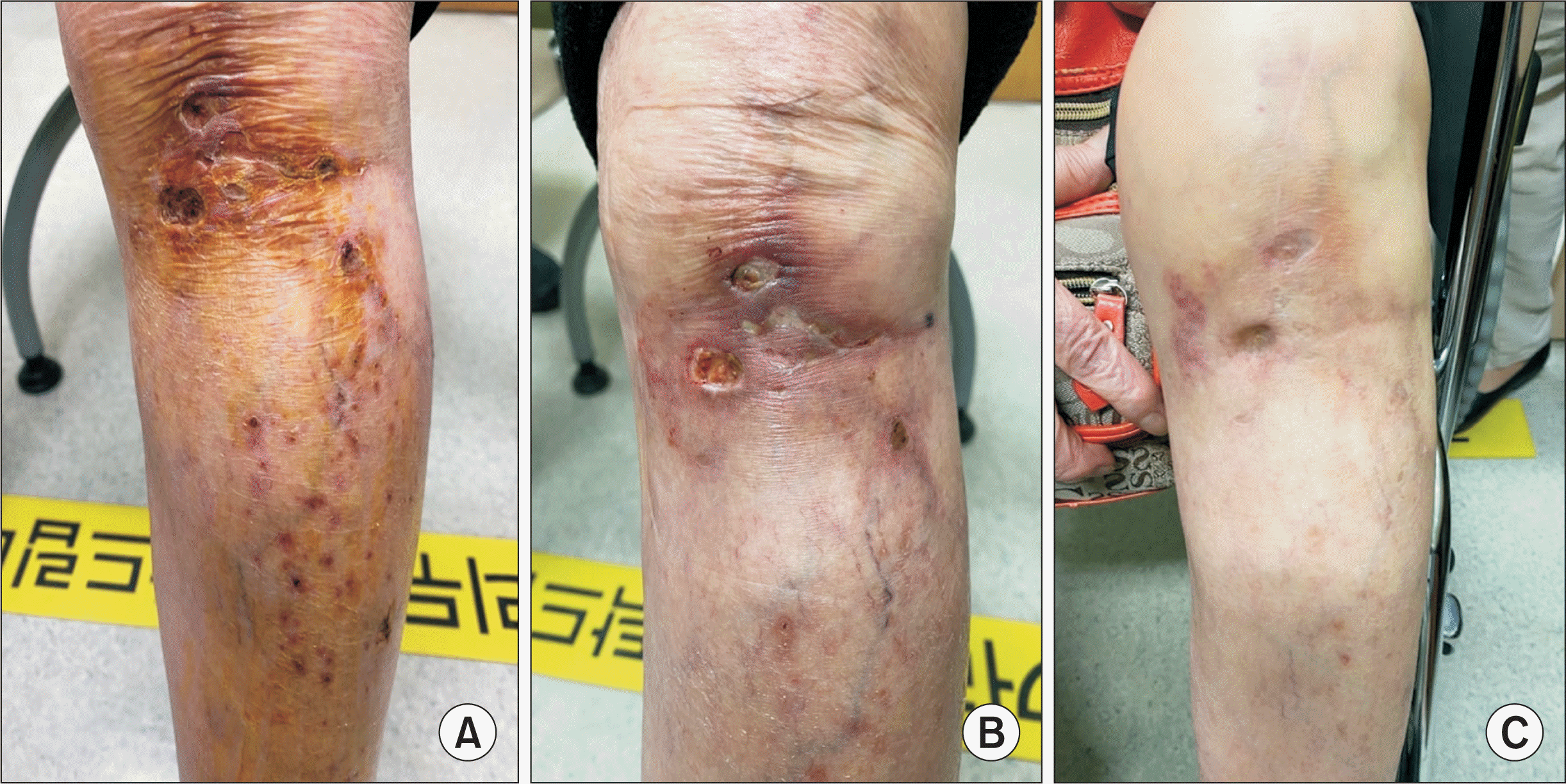
An 86-year-old female patient was diagnosed with seropositive rheumatoid arthritis (RA) in March 2019 and she also had approximately ten-year history of diabetes mellitus, hypertension, dyslipidemia, osteoporosis, and total knee replacement in her left knee joint in 2014. She had been treated with methotrexate, leflunomide, and prednisolone for RA, then tofacitinib was added for uncontrolled disease activity of RA in December 2019, symptoms and signs of arthritis subsided and she was treated on methotrexate, tofacitinib and methylprednisolone 2 mg daily thereafter. In April 2020, she developed erythematous papules, palpable purpura, and ulcers on the left lower leg, and visited the department of dermatology. There were no other systemic symptoms and signs, and laboratory tests were nonspecific. Suspicious of leukocytoclastic vasculitis (LCV), an allergic reaction, topical methylprednisolone was applied to have a good effect in 1~2 weeks. However, in December 2020 more severe skin lesions with the same morphology recurred that a punch biopsy was conducted (Figure 1A and 1B), and the result revealed thrombo-occlusive lesions in small vessels in the upper dermis with occlusion of the venules and capillaries with thrombotic clots, epidermal necrotic change, and relatively scarce infiltration of perivascular inflammatory cells, thus excluding vasculitis (Figure 1C and 1D). She had multiple risk factors for vascular diseases such as old age, diabetes mellitus, hypertension, dyslipidemia, the postoperative status of her left knee joint besides RA. Then, she was prescribed rivaroxaban, an oral anticoagulant with off-label usage similar to livedoid vasculitis along with supportive care such as dressing and improvement of skin lesions was shown in the next visit in 2 weeks, and she had complete resolution of lesions in 3 months (Figure 2) [1-4]. We excluded both deep vein and superficial vein thrombosis by Doppler ultrasonography in the lower extremities. After the biopsy results were reported, tofacitinib treatment was switched to leflunomide due to the possible association of tofacitinib and the patient’s small vascular thrombotic lesions. Her RA disease activity had maintained to a minimal level throughout the treatment course. The newly developed biopsy-proven thrombotic skin lesion with scarce inflammation in our patient brought up several differential diagnoses to be considered but was inconsistent with any existing specific thrombotic skin disease to our knowledge. And we tried differentiating her skin lesions from other entities including livedoid vasculopathy, LCV, and vasculitis. Her lesions were located unilaterally, however, skin lesions in livedoid vasculopathy were prone to be bilateral with some unilateral cases; and the gross appearance of the lesions was distinguished from those of livedoid vasculopathy which generally has atrophic, stellate, ivory to white plaques also known as “atrophie blanche”; the histologic findings of livedoid vasculopathy had characteristic endothelial proliferation with hyalinization even though thrombosis could commonly be accompanied [5]. In contrast to LCV or other various vasculitides, her lesions were unilateral with less perivascular inflammatory cell infiltration. Additionally, tofacitinib has been suspected to increase arterial or venous vascular events, including arterial thromboembolism, deep vein thrombosis, and pulmonary embolism, even though the current cutaneous thrombotic lesion had not been reported, the possibility of association still exists [6-9]. The superficial thrombo-occlusive vascular disease showed thrombosis in small vessels and the treatment could be anticoagulation.
We experienced a case of superficial thrombo-occlusive vascular disease in a patient with RA, along with other multiple cardiovascular risk factors successfully treated with rivaroxaban. When new skin lesions occur in patients with RA, a multidisciplinary approach should be prompted and diagnostic efforts for various differential diseases should be conducted.
This study was approved by the Institutional Review Board (KYUH IRB approval no. 2021-03-010-003). Informed consent was waived by the IRB.
Notes
AUTHOR CONTRIBUTIONS
Designing the research: S.P., M.K. Collecting patient data: S.P. Writing manuscript: S.P., M.K. Reviewing the literature: S.P., C.J., M.K. Performing and reading the skin biopsy: S.H.C. Dermatological advice: S.H.C. Advice in terms of vascular surgery: H.Y.R. Performing and reading the skin biopsy: G.H.L., G.J.P. Dermatological advice: G.H.L., G.J.P. Corresponding: M.K.
REFERENCES
1. Weishaupt C, Strölin A, Kahle B, Kreuter A, Schneider SW, Gerss J, et al. 2016; Anticoagulation with rivaroxaban for livedoid vasculopathy (RILIVA): a multicentre, single-arm, open-label, phase 2a, proof-of-concept trial. Lancet Haematol. 3:e72–9. DOI: 10.1016/S2352-3026(15)00251-3.

2. Beyer-Westendorf J, Schellong SM, Gerlach H, Rabe E, Weitz JI, Jersemann K, et al. 2017; Prevention of thromboembolic complications in patients with superficial-vein thrombosis given rivaroxaban or fondaparinux: the open-label, randomised, non-inferiority SURPRISE phase 3b trial. Lancet Haematol. 4:e105–13. DOI: 10.1016/S2352-3026(17)30014-5.

3. Lee JJ, Pope JE. 2014; A meta-analysis of the risk of venous thromboembolism in inflammatory rheumatic diseases. Arthritis Res Ther. 16:435. DOI: 10.1186/s13075-014-0435-y. PMID: 25253302. PMCID: PMC4207310.

4. Chung WS, Peng CL, Lin CL, Chang YJ, Chen YF, Chiang JY, et al. 2014; Rheumatoid arthritis increases the risk of deep vein thrombosis and pulmonary thromboembolism: a nationwide cohort study. Ann Rheum Dis. 73:1774–80. DOI: 10.1136/annrheumdis-2013-203380. PMID: 23926057.

5. Hairston BR, Davis MD, Pittelkow MR, Ahmed I. 2006; Livedoid vasculopathy: further evidence for procoagulant pathogenesis. Arch Dermatol. 142:1413–8. DOI: 10.1001/archderm.142.11.1413. PMID: 17116831.
6. Vallejo-Yagüe E, Weiler S, Micheroli R, Burden AM. 2020; Thromboembolic safety reporting of tofacitinib and baricitinib: an analysis of the WHO VigiBase. Drug Saf. 43:881–91. DOI: 10.1007/s40264-020-00958-9. PMID: 32533433.

7. Mease P, Charles-Schoeman C, Cohen S, Fallon L, Woolcott J, Yun H, et al. 2020; Incidence of venous and arterial thromboembolic events reported in the tofacitinib rheumatoid arthritis, psoriasis and psoriatic arthritis development programmes and from real-world data. Ann Rheum Dis. 79:1400–13. DOI: 10.1136/annrheumdis-2019-216761. PMID: 32759265. PMCID: PMC7569391.

8. Giménez Poderós T, Gallardo Borge S, Vazquez-Ferreiro P. 2020; Risk of venous thromboembolism associated with tofacitinib and baricitinib: a systematic review and indirect meta-analysis. Pharmacotherapy. 40:1248–64. DOI: 10.1002/phar.2472. PMID: 33064892.

9. Kotyla PJ, Engelmann M, Giemza-Stokłosa J, Wnuk B, Islam MA. 2021; Thromboembolic adverse drug reactions in Janus kinase (JAK) inhibitors: does the inhibitor specificity play a role? Int J Mol Sci. 22:2449. DOI: 10.3390/ijms22052449. PMID: 33671049. PMCID: PMC7957632.

Fig. 1
Her skin lesions with papules and ulcers were shown on her left leg unilaterally (A), and the biopsy site was marked (B). The crust formation (asterisks) was found in the epidermis layer, and there were diffuse infiltrates of inflammatory cells (black arrows) in the upper dermis (x100 high power field [HPF]) (C). High magnification showed neutrophils, lymphocytes (black arrowhead) in the upper dermis, and thrombosis (green arrowheads) in capillaries, and red blood cell extravasation (yellow arrowheads) could be seen (x200 HPF) (D).
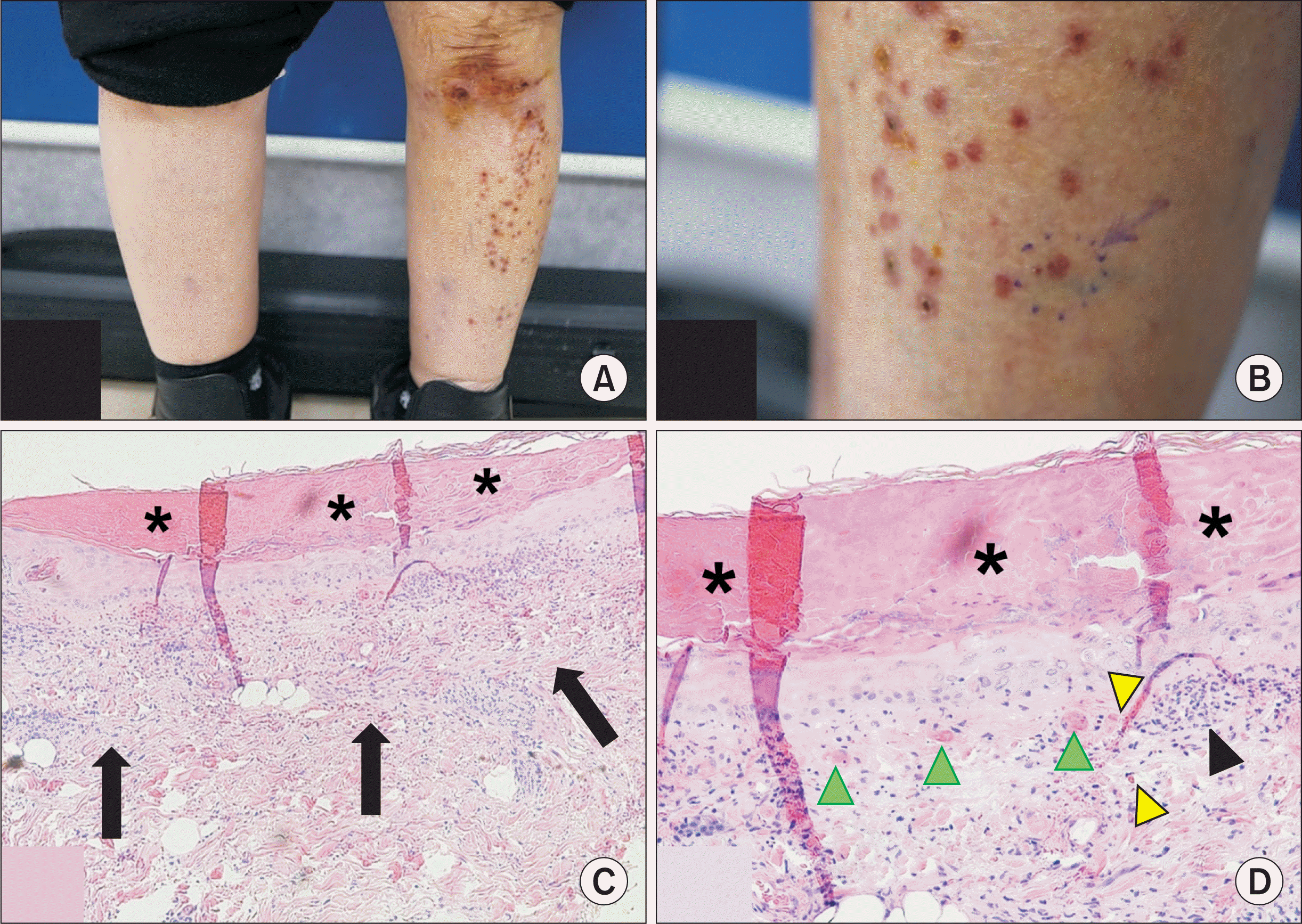
Fig. 2
The changes of the skin lesions. The initial appearance of the skin lesion at her second visit for worsening in December 2020 (A) and after a biopsy, she had prescribed rivaroxaban for superficial thrombo-occlusive vascular disease. In 2 weeks, the skin lesion which being treated with rivaroxaban (B), and in 3 months, she had complete resolution of symptoms (C).




 PDF
PDF Citation
Citation Print
Print



 XML Download
XML Download