Abstract
Background
On March 22, 2020, intense social distancing (SD) was implemented in Korea to prevent the spread of coronavirus disease 19 (COVID-19). This study examined the impact of SD on diabetes control in older adults with diabetes.
Methods
Adults aged 60 to 90 years with type 2 diabetes mellitus who were physically and mentally independent were recruited. Participants who had complete blood chemistry data from April to July 2019 (pre-SD era) and April to July 2020 (SD era) were enrolled. Data were obtained about physical activity, nutrition, sarcopenia, and psychological and mental health from questionnaires in April to July 2020. Calf circumference was measured.
Results
In total, 246 people (100 men, 146 women; mean age, 73.8±5.7 years) participated in this study. The levels of glycated hemoglobin (HbA1c, 7.4%±1.0% vs. 7.1%±0.8%, P<0.001), fasting glucose (142.2±16.7 mg/dL vs. 132.0±27.7 mg/dL, P<0.001), and body weight (62.6±9.4 kg vs. 61.8±10.1 kg, P<0.01) were higher in the SD era than in the pre-SD era. Total physical activity was lower in the SD era (2,584.6±2,624.1 MET-min/week–1 vs. 1,987.3±2,295.0 MET-min/week–1, P<0.001). A larger increase in HbA1c level was associated with increased body weight and decreased physical activity.
Conclusion
SD had negative effects on diabetes management in older adults with diabetes. Fasting glucose and HbA1c levels and body weight increased during the SD era. Participants with reduced physical activity gained more weight and had higher blood glucose levels. Given that the COVID-19 pandemic is ongoing, health professionals and diabetes educators should monitor changes in lifestyle factors in older adults with diabetes.
The first case of coronavirus disease 19 (COVID-19) in Korea was confirmed on January 19, 2020 [1], and the first death due to COVID-19 in Korea occurred on February 19, 2020 [1,2]. After the first COVID-19 outbreak in Daegu in mid-February 2020, the number of confirmed cases of COVID-19 increased rapidly and, by early March 2020, Korea had the second highest number of COVID-19 cases after China [1]. The Korean government raised the alert level from orange to red on February 23, 2020, and the World Health Organization declared a global pandemic outbreak of COVID-19 on March 11, 2020.
People with COVID-19 can have various clinical presentations. In an observational study in China, about 80% of patients with COVID-19 had mild respiratory symptoms, 14% had severe respiratory symptoms, and 5% had critical manifestations such as respiratory failure and/or multiorgan dysfunction [3]. However, 17% to 35% of patients hospitalized with COVID-19 required intensive treatment or admission to an intensive care unit because of hypoxemic respiratory failure [3,4]. The overall case fatality rate in a large observational study in China was 2.3%, but this rate was 8.0% in patients aged 70 to 79 years and 14.8% in patients aged 80 years and older [5]. COVID-19 causing severe manifestations or death is associated with several clinical characteristics such as advanced age, being male, and preexisting comorbid conditions such as cardiovascular disease, obesity and/or diabetes, hypertension, and cancer [6,7].
Because of the lack of effective antiviral agents to prevent the spread of COVID-19, social distancing (SD), wearing face masks, and self-hygiene have been implemented by the Korean government since February 2020. On March 22, 2020, the government implemented the first intense SD policy to prevent the spread of COVID-19. This significantly decreased public movement, as assessed by mobile big data, and the number of passengers at major subway stations in Seoul [8].
SD causes marked changes in the usual daily activities, which can lead to increases in sedentary behavior and/or less regular exercise, changes in eating patterns, and greater psychological burden in people with diabetes, all of which may have adverse effects on health, including glucose control [9]. Because they are more frail and vulnerable to social isolation and/or loneliness, SD may have more serious effects in older adults with diabetes who are at higher risk for severe morbidity and mortality of COVID-19, and these effects may exacerbate the underlying chronic disease [10,11].
The aim of this study was to examine the effects of SD on diabetes management in older adults with diabetes during the 2020 COVID-19 pandemic. We analyzed the effects of SD on blood glucose control, physical activity, nutrition, and stress in older adults with type 2 diabetes mellitus (T2DM).
From April to July 2020, we recruited older adults with T2DM aged 60 to 90 years seen in the outpatient clinic of Seoul National University Bundang Hospital (SNUBH). The inclusion criteria were diabetes duration of >2 years, blood glucose test results in the time frame April to July 2019, and regular follow-up at SNUBH. The exclusion criteria were type 1 diabetes mellitus or secondary diabetes, malignant tumors within 3 years, any medical condition affecting glucose metabolism, or chronic kidney disease of more than stage 3. We also excluded older adults with physical dysfunction (e.g., unable to walk without assistance) and cognitive dysfunction including mild cognitive impairment. This study was approved by the Institutional Review Board of SNUBH (B-2005/615-102). Among 400 eligible older adults with T2DM, 246 agreed to participate in this study. Written informed consent was obtained from all participants.
From each patient’s medical record, we obtained demographic and anthropometric data, including body weight, history of diabetes, microvascular and macrovascular complications, comorbidities, medication for diabetes and comorbidities, and blood chemistry test results for the periods April to July of 2019 (pre-SD era) and 2020 (SD era). The presence of diabetic retinopathy and peripheral neuropathy was determined by the ophthalmologists and by the endocrinologists, respectively. The presence of albuminuria was defined as a urinary albumin to creatinine ratio of ≥30 mg/g. Questionnaires about physical activity and nutrition based on the participants’ recall were completed for the two time periods, and questionnaires about sarcopenia and psychological and mental health were obtained in the SD era. We also measured calf circumference at the time of the questionnaires.
The Korean version of the International Physical Activity Questionnaire short form (IPAQ-SF) [12] was used to assess physical activity level in the pre-SD and SD eras. The IPAQ-SF is a publicly available instrument (http://www.ipaq.ki.se) that is often used to measure physical activity level. In this questionnaire, participants reported the number of minutes of vigorous and moderate intensity and walking physical activity for a typical week in the pre-SD area and for the past 7 days in the SD era. The minutes of each physical activity were converted to metabolic equivalent task minutes per week (MET/min/week–1) according to the IPAQ recommendations. Participants were classified into three groups according to the total amount of walking, moderate and vigorous intensity physical activities, expressed as MET/min/week–1: low activity (<600); moderate activity (600 to 2,999), and high activity (≥3,000) [13].
The Korean version of the strength, assistance walking, rising from a chair, climbing stairs, and falls (screening questionnaire for sarcopenia [SARC-F]) questionnaire was used to screen for sarcopenia [14]. The SARC-F comprises five items used to assess strength, assistance with walking, rising from a chair, climbing stairs, and falls. A sum of the scores for each item of ≥4 points predicts sarcopenia. We also measured the circumference of the protruding part of the calf (calf circumference) using a measuring tape in participants standing upright with the feet slightly apart and body weight evenly distributed on both feet [15].
The Mini Nutritional Assessment (MNA) tool [16] was used to evaluate the risk of malnutrition. Two items for the mid-arm and calf circumference were excluded from the evaluation because we did not collect these data in the pre-SD era. The maximum total score was adjusted to 28 points instead of 30. Malnutrition was defined as a score of <15 points and at risk of malnutrition as 15 to 21.5 points. Participants completed the MNA questionnaire regarding their current (SD era) and preSD nutritional status.
The Korean version of the Impact of Event Scale-Revised (IESR) [17] was used to evaluate psychological stress in relation to the COVID-19 pandemic. This tool comprises 22 items: eight evaluating intrusive symptoms (items 1, 2, 3, 6, 9, 14, 16, and 20), eight evaluating avoidance symptoms (items 5, 7, 8, 11, 12, 13, 17, and 22), and six evaluating hyperarousal symptoms (items 4, 10, 15, 18, 19, and 21). The score for each item ranges from 0 through 4. We classified the participants’ psychological stress according to the total score as minimal (<24), mild (24 to 32), moderate (33 to 36), and severe (≥37).
The Korean version of the Patient Health Questionnaire-9 (PHQ-9) [18] was used to assess mental health. This tool comprises nine items to assess depression, and the score for each ranges from 0 through 4. The severity of depression was classified using the total score as minimal (0 to 4), mild (5 to 9), moderate (10 to 14), moderately severe (15 to 19), and severe (≥20).
Continuous variables are expressed as mean±standard deviation, and categorical variables are expressed as numbers (%). To compare variables between the pre-SD and SD eras, a paired t-test was performed for continuous variables, and McNemar’s test was performed for categorical variables. Pearson’s chi-square test was performed to analyze the differences in frequencies between components according to the change in physical activity. Spearman’s rank correlational analysis was performed to identify correlations between changes in HbA1c level, body weight, total physical activity level, and IES-R and PHQ scores. For statistical analyses, IBM SPSS version 25.0 (IBM Corp., Armonk, NY, USA) was used, and P values <0.05 were considered to be significant.
Among the 400 eligible older adults, 246 agreed to participate in this observational study. Their baseline characteristics are shown in Table 1. The mean age was 73.8±5.7 years, 40.7% were men, and their mean duration of diabetes was 17.7±8.8 years. Body weight was higher in the SD era than in the pre-SD era, but body mass index did not change. Among the 231 participants with a measured calf circumference, 82 (35.4%) had a smaller calf circumference and 12 (6.2%) had a higher SARC-F (≥4) according to the Asian Working Group for Sarcopenia 2019 algorithm [11]. The mean values for blood pressure and serum levels of fasting glucose, HbA1c, total cholesterol, high-density lipoprotein cholesterol, low-density lipoprotein cholesterol, and creatinine were significantly higher in the SD era than in the pre-SD era. Except for retinopathy in older adults with diabetes, the frequencies of microvascular and macrovascular complications did not differ between the two eras. The type of glucose-lowering therapy also did not differ between the two eras.
A social history was obtained for 179 participants. Employment status changed from the pre-SD to SD era for only eight participants: in the pre-SD era, 39 participants were employed and 140 were unemployed; in the SD era, 36 participants were employed.
The mean minutes per week of vigorous, moderate, walking, and total physical activity in the pre-SD and SD eras are shown in Table 2. All levels of physical activity decreased from the preSD to SD era: vigorous (–105.6 min/week, –27.7%), moderate (–308.5 min/week, –41.1%), walking (–183.3 min/week, –12.6%), and total (–597.3 min/week, –23.1%). The percentages of participants who decreased their physical activity did not differ significantly between men and women. As expected, in the pre-SD era, muscular strength and power and physical activity level were greater in the youngest age group (60 to 69 years old) than in the two older age groups (70–79 and 80–89 years). However, a higher percentage with high physical activity in the youngest age group changed to the moderate or low activity groups from the pre-SD to SD era (Supplementary Table 1).
The MNA score was higher in older adults with diabetes in the SD era than in the pre-SD era (Supplementary Table 2). The number of participants with malnutrition or risk of malnutrition decreased from the pre-SD to the SD era. The MNA score increased in 180 of the 246 participants (73.2%) from the pre-SD to SD era.
Psychological stress during the SD era was measured using the IES-R scale. The mean score was 6.4±6.6. Nearly all participants (97.2%) exhibited minimal psychological impact (IES-R score <24) and only four (1.6%) had a more than moderate level of psychological stress (IES-score ≥33) (Table 3).
The PHQ-9 score was available for 233 participants, and the mean score was 3.1±3.6. Most participants had minimal (73.7%) or mild (22.0%) depressive symptoms; only 10 had moderate (2.1%) or moderately severe (2.1%) depressive symptoms. None of the participants reported severe depression in the SD era.
The increases in body weight and fasting glucose and HbA1c levels were larger in participants whose physical activity levels decreased compared with those whose physical activity level did not decrease (Supplementary Table 3). The PHQ-9 and IES-R scores did not differ significantly according to the change in physical activity level. The frequencies of sarcopenia, as shown by SARC-F and SARC-F+calf circumference (SARC-Calf), also did not differ according to the change in physical activity level. The changes in fasting glucose and HbA1c levels correlated positively with the changes in body weight and negatively with the change in total physical activity level (Table 4).
The patterns of change in HbA1c levels according to the changes in body weight and physical activity level are shown in Fig. 1. The HbA1c level increased more in those whose body weight increased (Fig. 1A) and physical activity level decreased (Fig. 1B). However, the change of HbA1c did not differ significantly according to the severity of psychological stress and depressive symptoms. The HbA1c level increased by >2.0% between the pre-SD and SD era in eight participants, and the explanations for their increased HbA1c level are listed in Supplementary Table 4. Most of those whose HbA1c increased reported an unhealthy diet and decreased physical activity.
This study demonstrates that SD during the COVID-19 pandemic had negative effects on diabetes management in older adults with diabetes, as shown by decreases in physical activity levels, increased body weight, and deterioration of glucose control. The amounts of total physical activity and vigorous and moderate physical activity decreased more than that of walking physical activity from the pre-SD to SD era. Fasting glucose and HbA1c levels and body weight increased more in participants whose physical activity level decreased than in those whose physical activity level did not decrease. The youngest age group (60 to 69 years) had a greater high physical activity level in the pre-SD era, but more of this age group were likely to decrease their activity to the moderate or low level during the SD era. By contrast, the changes in fasting glucose and HbA1c levels did not differ significantly between age groups. The presence of sarcopenia was not related to physical activity level, and SD was not associated with the risk of malnutrition in older adults. The psychological stress and depressive symptoms were minimal or mild in these participants and were not significantly associated with changes in physical activity, fasting glucose, and HbA1c levels.
A recent study in Daegu, Korea, showed that the HbA1c level increased during SD in people with T2DM, especially in younger people (<50 years old) and those with an HbA1c level <7.0% [19]. However, that study did not include data on body weight, physical activity, and psychological assessment. Another study of 203 Japanese people (mean age, 67.4±11.3 years) with T2DM reported increased body weight and HbA1c level and decreased exercise during SD compared with the previous 3 months [9]. Although the questionnaire used in the Japanese study was simple, it showed that increased food and snack intake, and decreased exercise because of stress worsened the glucose control. Our data indicate that body weight and HbA1c levels increased during SD and these changes were associated with decreased physical activity in older adults with diabetes. However, the psychological stress and depressive symptoms reported in our study were minimal or mild and were not associated with changes in glucose control and body weight.
An international online survey in April 2020 showed that SD decreased all physical activity levels and increased out-of-control eating and unhealthy food and snack intakes [20]. An earlier report from Korea showed an 11% increase in the amounts of delivered food, mostly fast foods such as pizza, hamburgers, and fried chicken, in early February 2020 compared with before the COVID-19 pandemic [21]. In the present study, we used the MNA to assess the risk of malnutrition and nutritional status of older adults with diabetes because these are important health issues for older adults. The MNA score increased in most participants, which indicated that the risk of malnutrition did not increase during SD in these participants. Further studies on dietary pattern or eating behaviors in older adults are warranted.
The COVID-19 pandemic is thought to have had a negative effect on mental health and well-being, including depression, posttraumatic stress disorder, and substance use disorder [10]. An online survey in the USA in February 2020 reported greater psychological distress during COVID-19 compared with 2018 [22]. In that study, the symptoms of psychological distress were highest among young adults and lowest in older adults. Other studies have reported that older adults are less negatively affected by COVID-19 compared with other age groups and that COVID-19 increases loneliness, although mental health level remains unchanged in community-dwelling older adults [23,24]. Our results are consistent with these earlier findings.
Our study has some limitations. First, physical activity was measured using the IPAQ-SF, which provides an indirect measure of physical activity that is based on participant self-report. Second, the study participants were recruited in a single university hospital located in Gyeonggi-do, Korea, and included only physically and mentally independent people, who may not represent the population of older Korean adults with T2DM. Third, because the data were collected during the first period of intense SD, our findings reflect only the short-term effects of SD. Further studies are needed to examine the long-term effects of SD.
In conclusion, COVID-19 SD had negative effects on diabetes management in older adults with diabetes. Fasting glucose and HbA1c levels and body weight increased after SD was introduced. Participants who reduced their physical activity levels gained more weight and had higher blood glucose levels. Given that the COVID-19 pandemic is ongoing, health professionals and diabetes educators should monitor changes in lifestyle and body weight in older adults with diabetes.
Supplementary Materials
Supplementary materials related to this article can be found online at https://doi.org/10.4093/dmj.2021.0096.
Supplementary Table 1.
Change in physical activity level from pre-SD to SD era according to age group
Supplementary Table 2.
Changes in nutritional status from the pre-SD to SD era
Supplementary Table 3.
Demographic, metabolic, and psychological variables according to the changes in total physical activity level
Supplementary Table 4.
Participants’ explanations of the reason for an increase in HbA1c level >2.0%
Notes
ACKNOWLEDGMENTS
We thank Song YJ, Kim HR, Kim KH, and Lim SA (Seoul National University Bundang Hospital) for assistance.
REFERENCES
1. Korean Society of Infectious Diseases; Korean Society of Pediatric Infectious Diseases; Korean Society of Epidemiology; Korean Society for Antimicrobial Therapy; Korean Society for Healthcare-associated Infection Control and Prevention; Korea Centers for Disease Control and Prevention. Report on the epidemiological features of coronavirus disease 2019 (COVID-19) outbreak in the Republic of Korea from January 19 to March 2, 2020. J Korean Med Sci. 2020; 35:e112.
2. Korean Society of Infectious Diseases and Korea Centers for Disease Control and Prevention. Analysis on 54 mortality cases of coronavirus disease 2019 in the Republic of Korea from January 19 to March 10, 2020. J Korean Med Sci. 2020; 35:e132.
3. Wiersinga WJ, Rhodes A, Cheng AC, Peacock SJ, Prescott HC. Pathophysiology, transmission, diagnosis, and treatment of coronavirus disease 2019 (COVID-19): a review. JAMA. 2020; 324:782–93.
4. Myers LC, Parodi SM, Escobar GJ, Liu VX. Characteristics of hospitalized adults with COVID-19 in an integrated health care system in California. JAMA. 2020; 323:2195–8.

5. Wu Z, McGoogan JM. Characteristics of and important lessons from the coronavirus disease 2019 (COVID-19) outbreak in China: summary of a report of 72 314 cases from the Chinese Center for Disease Control and Prevention. JAMA. 2020; 323:1239–42.
6. Jordan RE, Adab P, Cheng KK. COVID-19: risk factors for severe disease and death. BMJ. 2020; 368:m1198.

7. Goyal P, Choi JJ, Pinheiro LC, Schenck EJ, Chen R, Jabri A, et al. Clinical characteristics of COVID-19 in New York City. N Engl J Med. 2020; 382:2372–4.

8. Park IN, Yum HK. Stepwise strategy of social distancing in Korea. J Korean Med Sci. 2020; 35:e264.

9. Munekawa C, Hosomi Y, Hashimoto Y, Okamura T, Takahashi F, Kawano R, et al. Effect of coronavirus disease 2019 pandemic on the lifestyle and glycemic control in patients with type 2 diabetes: a cross-section and retrospective cohort study. Endocr J. 2021; 68:201–10.

10. Galea S, Merchant RM, Lurie N. The mental health consequences of COVID-19 and physical distancing: the need for prevention and early intervention. JAMA Intern Med. 2020; 180:817–8.
11. Lim WS, Liang CK, Assantachai P, Auyeung TW, Kang L, Lee WJ, et al. COVID-19 and older people in Asia: Asian Working Group for Sarcopenia calls to actions. Geriatr Gerontol Int. 2020; 20:547–58.
12. Oh JY, Yang YJ, Kim BS, Kang JH. Validity and reliability of Korean version of International Physical Activity Questionnaire (IPAQ) short form. J Korean Acad Fam Med. 2007; 28:532–41.
13. Maugeri G, Castrogiovanni P, Battaglia G, Pippi R, D’Agata V, Palma A, et al. The impact of physical activity on psychological health during COVID-19 pandemic in Italy. Heliyon. 2020; 6:e04315.

14. Kim S, Kim M, Won CW. Validation of the Korean version of the SARC-F questionnaire to assess sarcopenia: Korean frailty and aging cohort study. J Am Med Dir Assoc. 2018; 19:40–5.

15. Chen LK, Woo J, Assantachai P, Auyeung TW, Chou MY, Iijima K, et al. Asian Working Group for Sarcopenia: 2019 consensus update on sarcopenia diagnosis and treatment. J Am Med Dir Assoc. 2020; 21:300–7.

16. Guigoz Y, Lauque S, Vellas BJ. Identifying the elderly at risk for malnutrition. The Mini Nutritional Assessment. Clin Geriatr Med. 2002; 18:737–57.
17. Eun HJ, Kwon TW, Lee SM, Kim TH, Choi MR, Cho SJ. A study on reliability and validity of the Korean version of Impact of Event Scale-Revised. J Korean Neuropsychiatr Assoc. 2005; 44:303–10.
18. Han C, Jo SA, Kwak JH, Pae CU, Steffens D, Jo I, et al. Validation of the Patient Health Questionnaire-9 Korean version in the elderly population: the Ansan Geriatric study. Compr Psychiatry. 2008; 49:218–23.

19. Park SD, Kim SW, Moon JS, Lee YY, Cho NH, Lee JH, et al. Impact of social distancing due to coronavirus disease 2019 on the changes in glycosylated hemoglobin level in people with type 2 diabetes mellitus. Diabetes Metab J. 2021; 45:109–14.

20. Ammar A, Brach M, Trabelsi K, Chtourou H, Boukhris O, Masmoudi L, et al. Effects of COVID-19 home confinement on eating behaviour and physical activity: results of the ECLB-COVID19 International Online Survey. Nutrients. 2020; 12:1583.
21. Lim S, Lim H, Despres JP. Collateral damage of the COVID-19 pandemic on nutritional quality and physical activity: perspective from South Korea. Obesity (Silver Spring). 2020; 28:1788–90.

22. McGinty EE, Presskreischer R, Han H, Barry CL. Psychological distress and loneliness reported by US adults in 2018 and April 2020. JAMA. 2020; 324:93–4.

23. Czeisler ME, Lane RI, Petrosky E, Wiley JF, Christensen A, Njai R, et al. Mental health, substance use, and suicidal ideation during the COVID-19 pandemic: United States, June 24-30, 2020. MMWR Morb Mortal Wkly Rep. 2020; 69:1049–57.
Fig. 1.
The pattern of changes in glycated hemoglobin (HbA1c) levels in the two groups according to changes in body weight and physical activity. Box and whisker plots of HbA1c levels. Whiskers represent the 5th and 95th percentiles. (A) Change in body weight. (B) Change in physical activity. aP<0.05, t-test.
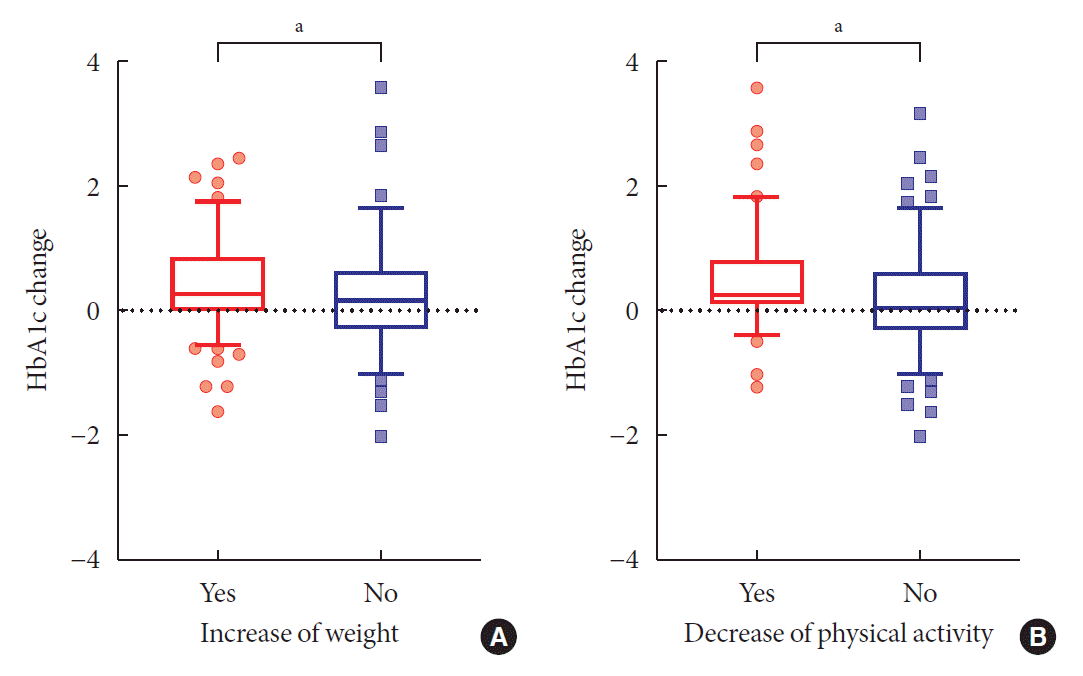
Table 1.
Characteristics of participants in the pre-SD and SD eras
Table 2.
Changes in physical activity from the pre-SD to SD era
Table 3.
Psychological stress assessed by the Impact of Event Scale-Revised
Table 4.
Correlations between changes in HbA1c level, body weight, total physical activity level, and IES-R and PHQ-9 scores from the pre-SD to SD era




 PDF
PDF Citation
Citation Print
Print



 XML Download
XML Download