TO THE EDITOR: Neonatal alloimmune thrombocytopenia (NAIT) is the most important cause of thrombocytopenia in term neonates. It was estimated to occur at a frequency of 1 in 1,000–2,000 live births [1]. Platelet destruction is caused by a maternal antibody directed against a fetal human platelet antigen (HPA) inherited from the father and lacking in the platelets of the mother. In the Caucasian population, 80% alloimmunization occurs predominantly due to antibodies to the HPA-1a antigen [1]. Although less common, the HPA-15 antigen system is also implicated in serologically confirmed NAIT cases [2]. The HPA-15a15b system is located on the glycosyl-phosphatidylinositol-anchored protein, CD109, and is defined by the single amino acid substitution of Ser703Tyr (Ser682Tyr of mature CD109 protein) [3]. The diagnosis of NAIT can be established by documenting the presence of a platelet-specific antigen incompatibility between mother and infant (or mother and father) and the presence of maternal antiplatelet allo-antibodies directed against the incompatible HPA antigen [4]. The monoclonal antibody-specific immobilization of platelet antigen (MAIPA) assay is considered as the ‘gold-standard’ test to detect maternal anti-HPA antibodies [4]. However, it is not available for routine use in India. Platelet cross-match, which is commonly used to provide platelet transfusion support in platelet refractoriness, may be considered as an alternative strategy to manage emergency cases of NAIT in resource-limited facilities where a platelet-donor registry is still unavailable [5].
In 2015, heterozygous mutations in the MECOM (MDS1 and EVI1 complex locus) were first identified as a cause of amegakaryocytic thrombocytopenia in neonates [6]. There are many novel mutations in the MECOM gene that have been identified recently, and a new name, ‘MECOM-associated syndrome,’ has been proposed for this disease [7]. Herein, we report a rare case of NAIT associated with HPA-15b incompatibility in a baby who was managed initially with the transfusion of cross-matched platelets, but due to persistent transfusion-dependent thrombocytopenia and inadequate response to appropriate therapy such as IVIG, whole exome sequencing was performed, which revealed the presence of a de novo pathogenic MECOM variant.
A 2.69 kg male baby with a body surface area of 0.2 m2 was born through an elective cesarean section at term after an uneventful pregnancy in a tertiary care hospital. At birth, the baby was noted to have a few ecchymotic patches on the trunk. However, within a few hours, the baby became pale and soon developed multiple ecchymotic patches on the trunk, and had evidence of mucosal bleeds in the form of bloody gastro-intestinal aspirates and subconjunctival hemorrhages. There was no dysmorphism, microcephaly, or organomegaly. The baby was the firstborn child of a non-consanguineous couple with no family history of hematological disorders. The mother was healthy throughout the antenatal period with normal platelet counts and no features of connective tissue disease. She also had an intact spleen. There were no clinical or laboratory features of neonatal sepsis, intrauterine viral infections, or inherited syndromes in the baby. There was no abnormality detected in the coagulation profile on day 1, the parameters of the liver function test were within their normal ranges, and ultrasound studies revealed no major internal hemorrhage. The platelet count was 8×109/L, and the hemoglobin (Hb) level was 10.1 g/dL at first testing, although it plummeted to 4.4 g/dL on day 2 because of bleeding. The blood group of the baby was ‘O’ rhesus positive, mother was ‘A’ rhesus positive, and father was ‘O’ rhesus positive. On day 1, the baby was transfused with 20 mL/kg ABO-specific uncross-matched random donor platelets and administered 1 g/kg of IVIG. The next day, the baby received 15 mL/kg O-positive packed red cell transfusion due to low Hb levels. The platelet count improved to 135×109/L on day 2 but decreased gradually to 46×109/L on day 5; hence, a second dose of IVIG was administered. The platelet count decreased further to 20×109/L by day 7 without any bleeding manifestations; therefore, a further 0.4 g/kg IVIG was administered. The baby’s blood sample, along with the blood samples of his parents, was sent to a platelet serology laboratory, and another sample was sent for HPA genotyping of the baby and parents to detect HPA incompatibility. Table 1 summarizes all the results of platelet cross-matching, platelet antibody screening, and HPA genotyping. HPA genotyping was performed for HPA-1, 2, 3, 4, 5, 6, and 15 systems by DNA sequencing at the national referral laboratory. Platelet antibody testing was performed according to manufacturers’ instructions using solid-phase adherence with an IgG platelet antibody detection and cross-match system (Capture-P, Immucor, Peachtree Corners, GA, USA), which utilizes fresh platelets bound to microplate wells from single group O donors [8]. There was no anti-HLA antibody detected in the mother when tested using Luminex technology. The chance of obtaining a compatible platelet unit for transfusion increased with the age of the baby, suggesting a gradual clearance of maternal-derived alloantibodies from the baby’s circulation. All platelets that were administered for transfusion starting from day 7 were O group random donor cross-matched compatible platelets, leukodepleted, and irradiated. The 24 hr corrected count increment (CCI) after transfusion of cross-matched platelets on day 7, day 23, and day 28 were 10000, 11667, and 13333, respectively. Fig. 1 shows the day-wise changes observed in the platelet counts. Because of the inadequate response to standard therapy for NAIT, such as IVIG, whole exome sequencing was performed after day 30, which revealed the presence of a pathogenic MECOM variant in the baby. No such mutation was found in parents, suggesting a de novo variant in this case. A heterozygous four-base pair deletion in exon 16 of the MECOM gene that results in a frameshift and premature truncation of the protein 33 amino acids downstream of codon 1043 was detected (Table 2). The p.Gln1043 IlefsTer33 variant has not been reported before in the 1000 genomes, gnomAD, and internal databases. No skeletal abnormality was detected in this case. A bone marrow examination was performed before initiating a plan for allogeneic stem cell transplantation in this baby, which revealed a lack of megakaryocytes in the marrow. The risk of subsequent pregnancies and post-transfusion purpura in mothers was explained to the family in detail.
The most important lesson learned from this case is that the MECOM-associated syndrome does not rule out the presence of concomitant NAIT, as they are not mutually exclusive conditions. During platelet cross-matching, the incompatibility observed between the baby and the parents was the diagnostic pointer in this case, which raised the suspicion of NAIT even before the availability of HPA-genotyping results. The platelet serology results and HPA genotyping almost confirmed the HPA incompatibility in this case. A subsequent final confirmation by MAIPA could be the last step of the diagnostic algorithm; unfortunately, it could not be performed due to the unavailability of the assay. NAIT due to anti-HPA-15b incompatibility has never been reported in the Indian population. In NAIT, where HPA-15b is the only antibody involved, severe and persistent anemia can be detected without any evident etiology [9]. CD109 is also expressed on CD34+ progenitor cells, which might be a reason for thrombocytopenia and anemia in the presence of anti-HPA-15 antibodies [9]. Apart from cross-matched platelets, washed-irradiated maternal platelets might be another option for transfusion in NAIT cases where a platelet-donor registry is unavailable [4]. However, it was not considered in this case, as the mother was unable to move from the primary hospital due to the ongoing COVID-19 pandemic and lockdown crisis. Although it is recommended that transfusion should not be delayed in NAIT if the appropriate antigen-negative platelets are unobtainable [4, 10] cross-matched platelets might be considered a better alternative than routine random donor platelets. The platelet transfusion threshold, in this case, was in line with the BCSH guidelines [10]. Heterozygous mutations in the MECOM gene are associated with different clinical manifestations, starting from inherited bone marrow failure syndrome to skeletal abnormalities [7]. The reason for the excessive platelet transfusion requirement in this baby was finally understood during the course of the disease after the identification of the de novo MECOM variant.
Therefore, we conclude that in resource-limited settings, platelet cross-match might be considered as an alternative strategy for the diagnosis and management of NAIT cases until a specific diagnostic assay such as MAIPA or structured platelet-donor registry is available and the evaluation of MECOM variants by whole exome sequencing should be integrated into the diagnostic algorithm in cases of persistent transfusion-dependent neonatal thrombocytopenia.
ACKNOWLEDGMENTS
We are thankful to Dr. BP. Kulkarni from the NIIH, Mumbai, India for performing HPA genotyping and Medgenome laboratories, Bangalore, India for conducting whole exome sequencing.
REFERENCES
1. Kjeldsen-Kragh J, Killie MK, Tomter G, et al. 2007; A screening and intervention program aimed to reduce mortality and serious morbidity associated with severe neonatal alloimmune thro-mbocytopenia. Blood. 110:833–9. DOI: 10.1182/blood-2006-08-040121. PMID: 17429009.

2. Mandelbaum M, Koren D, Eichelberger B, Auerbach L, Panzer S. 2005; Frequencies of maternal platelet alloantibodies and autoantibodies in suspected fetal/neonatal alloimmune thrombocytopenia, with emphasis on human platelet antigen-15 alloimmunization. Vox Sang. 89:39–43. DOI: 10.1111/j.1423-0410.2005.00662.x. PMID: 15938738.

3. Curtis BR, McFarland JG. 2014; Human platelet antigens - 2013. Vox Sang. 106:93–102. DOI: 10.1111/vox.12085. PMID: 24102564.

4. Peterson JA, McFarland JG, Curtis BR, Aster RH. 2013; Neonatal alloimmune thrombocytopenia: pathogenesis, diagnosis and management. Br J Haematol. 161:3–14. DOI: 10.1111/bjh.12235. PMID: 23384054. PMCID: PMC3895911.


5. Venkatesh HA, Paul A, Kantharaj A, Chandrashekar S. 2019; A case report of neonatal alloimmune thrombocytopenia: the utility of platelet crossmatch studies in the diagnosis and management. Glob J Transfus Med. 4:234–6. DOI: 10.4103/GJTM.GJTM_50_19.
6. Niihori T, Ouchi-Uchiyama M, Sasahara Y, et al. 2015; Mutations in MECOM, encoding oncoprotein EVI1, cause radioulnar synostosis with amegakaryocytic thrombocytopenia. Am J Hum Genet. 97:848–54. DOI: 10.1016/j.ajhg.2015.10.010. PMID: 26581901. PMCID: PMC4678429.


7. Germeshausen M, Ancliff P, Estrada J, et al. 2018; MECOM-associated syndrome: a heterogeneous inherited bone marrow failure syndrome with amegakaryocytic thrombocytopenia. Blood Adv. 2:586–96. DOI: 10.1182/bloodadvances.2018016501. PMID: 29540340. PMCID: PMC5873238.


8. Juskewitch JE, Norgan AP, De Goey SR, et al. 2017; How do I … manage the platelet transfusion-refractory patient? Transfusion. 57:2828–35. DOI: 10.1111/trf.14316. PMID: 28960321.

9. Nogues N, Garcia A, Julia MR, et al. 2006; Anti-HPA15 (gov) antibodies detected in two cases of neonatal alloimmune trombocytopenia and in one case of immune refractoriness to platelet transfusion. Vox Sang. 91(Suppl 2):16–17. DOI: 10.1111/j.1423-0410.2006.00801.x.
10. Gibson BE, Todd A, Roberts I, et al. 2004; Transfusion guidelines for neonates and older children. Br J Haematol. 124:433–53. DOI: 10.1111/j.1365-2141.2004.04815.x. PMID: 14984493.

Fig. 1
Day-wise changes observed in platelet counts after transfusion of cross-matched platelets and IVIG.
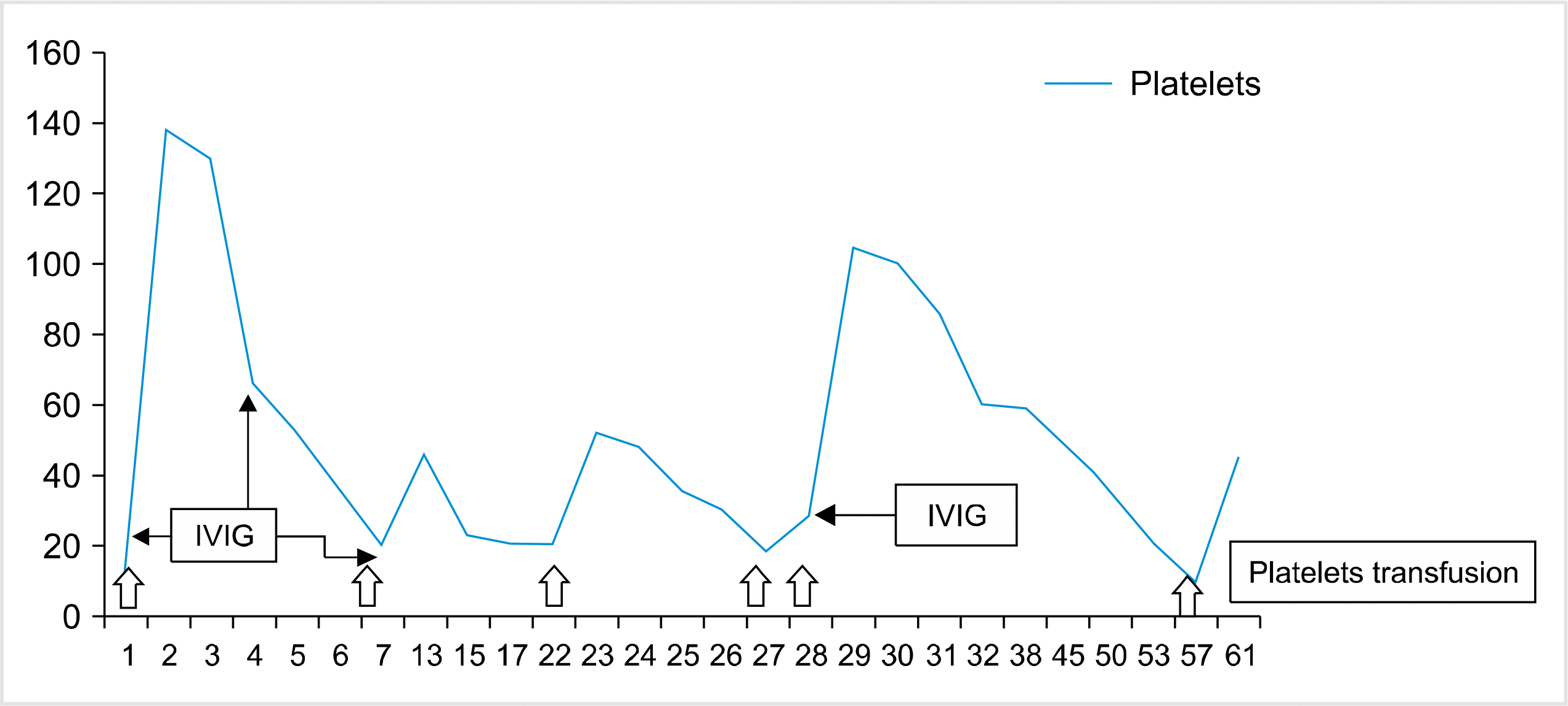
Table 1
Platelet cross-match, platelet antibody screening and HPA genotyping results.
Table 2
Whole exome sequencing results in baby.




 PDF
PDF Citation
Citation Print
Print


 XML Download
XML Download