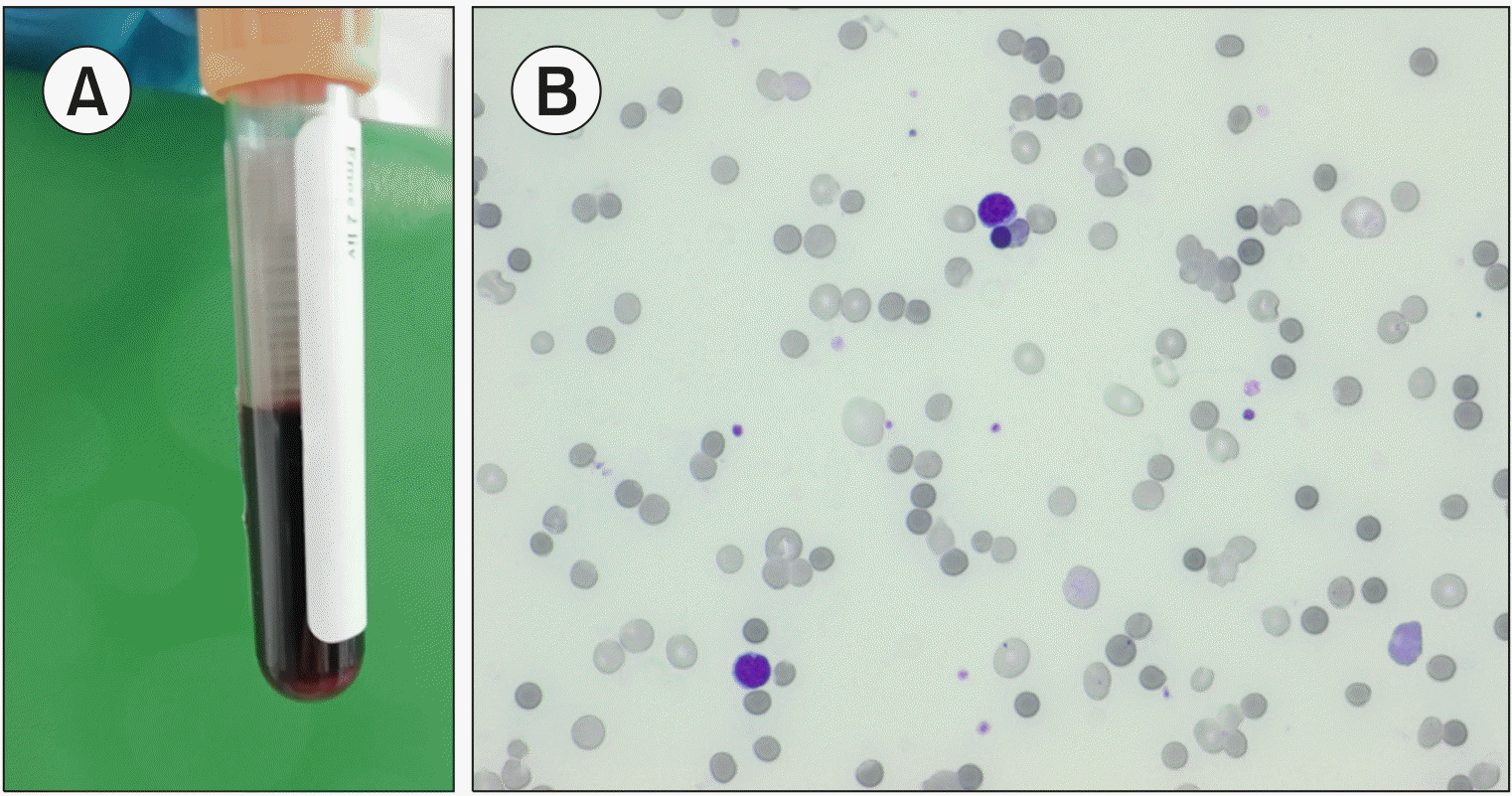
A 64-year-old woman with fever and weakness was recently administered the first course of chemotherapy for acute myeloid leukemia. Tests of a blood sample at our emergency laboratory showed the following: red blood cell (RBC) count, 0.91×1012/L; hematocrit, 0.07 (L/L); hemoglobin concentration, 3.6 g/dL; mean corpuscular volume, 76.9 fL; mean corpuscular hemoglobin, 39.6 pg; mean corpuscular hemoglobin concentration, 514 g/L; RBC distribution width, 16.8%; RBC distribution width with standard deviation, 55.8 fL; white blood cell count, 0.87×109/L; platelet count, 28×109/L; nucleated RBC count, 0.56×109/L; and reticulocyte count, 181.2×109/L. Plasma and serum samples appeared grossly hemolyzed, and all biochemical tests failed (A). Blood film showed many microspherocytes, nucleated RBCs, rare neutrophils, and the absence of blasts (B). Numerous spherocytes are observed in hereditary spherocytosis, immune hemolytic anemia, burns, toxic damage, and infections. Clostridium perfringens α toxin hydrolyzes phospholipids in RBC membranes, causing spherocytosis and hemolysis. Herein, a negative Coombs’ test result and C. perfringens identification in blood cultures led to accurate diagnosis. Prompt concentrated RBC transfusions and vancomycin antibiotic therapy avoided an otherwise likely and rapidly fatal outcome. Massive hemolysis, masking critical care settings, may be incorrectly considered an error during sample collection. Blood smear revisions aid differential diagnosis.




 PDF
PDF Citation
Citation Print
Print


 XML Download
XML Download