Dear Editor,
Progression to promyelocytic blast phase (BP) in chronic myeloid leukemia (CML), BCR-ABL1-positive is very rare, and only few cases have been reported since the introduction of Tyrosine Kinase Inhibitor (TKI) therapy [1-3]. However, there are no studies on the important considerations for an accurate diagnosis. We report the case of a patient with promyelocytic BP of CML after short-term TKI therapy and provide a review on the diagnosis. The Institutional Review Board of Soonchunhyang University Cheonan Hospital, Chungcheongnam-do, Korea, approved this study (file No. 2020-10-036) and exempted the need for informed consent as there was no identifying information and no harm caused to the patient.
A 35-year-old male patient with leukocytosis (60.55 × 109/L) was admitted to Soonchunhyang University Cheonan Hospital in March 2017. Bone marrow (BM) study revealed hypercellularity with myeloid and megakaryocytic hyperplasia.Chromosomal analysis revealed 46,XY,t(9;22)(q34;q11.2)[20]. The quantity of major BCR-ABL1 fusion transcript was 30.02% (Table 1). The patient was diagnosed as having CML, chronic phase (CP). However, he refused TKI therapy.
Two years later (in April 2019), his peripheral blood (PB) smear revealed marked leukocytosis (164.42 × 109/L), anemia, and thrombocytosis, with 2% blasts and left-shifted neutrophilic maturation. Newly developed hepatosplenomegaly was detected. BM finding was similar to the previous results, but diffuse myelofibrosis was additionally detected. The result of chromosomal analysis was same as before, but the quantity of BCR-ABL1 fusion transcript increased to 96.17%. The patient was started on dasatinib treatment (100 mg once daily). His leukocyte count normalized during the first month of therapy but gradually increased thereafter.
After two months of dasatinib therapy, the patient complained of oral bleeding. PB smear showed leukocytosis with 97% abnormal promyelocytes, anemia, and thrombocytopenia. The disseminated intravascular coagulation (DIC) score calculated using the International Society on Thrombosis and Haemostasis scoring system was 9 (Table 1) [4]. BM study revealed 96.20% abnormal promyelocytes (Fig. 1A). Immunophenotype of abnormal promyelocytes was consistent with acute promyelocytic leukemia (APL). Chromosomal analysis revealed 46,XY,t(9;22)(q34; q11.2),t(15;17)(q24;q21)[20]. The quantity of BCR-ABL1 fusion transcript was 53.22%. Multiplex reverse transcriptase-PCR and interphase fluorescence in-situ hybridization (FISH) detected both major BCR-ABL1 and PML-RARA fusion transcripts (Fig. 1B-1D). The patient was diagnosed as having promyelocytic BP of CML and received induction and maintenance chemotherapy. However, complete remission of promyelocytic BP was not achieved, and stem cell transplantation was considered.
We found three reported cases of promyelocytic BP of CML with BCR-ABL1 and PML-RARA rearrangements that have been reported after the introduction of TKI therapy (Table 1) [1-3]. All reported patients were primarily diagnosed as having CML, CP and immediately started on TKI therapy for which they showed good response. The time to progression into promyelocytic BP after starting treatment varied from six to 16 months. In contrast, our patient’s condition aggravated to promyelocytic BP just two months after starting treatment; therefore, the time was insufficient to evaluate the response to TKI therapy. Most patients showed increased hypergranular abnormal promyelocytes, and immunophenotypes were typical for APL. The quantities of fusion transcripts of BCR-ABL1 and PML-RARA were variable, but the proportion of fusion signals in FISH were high in all cases.
The following mechanisms have been suggested for disease progression from CML, CP to common BP despite TKI therapy: competitive advantage to Philadelphia-negative cells with genetic instability, chromosomal aberrations, and mutations of tumor suppressor genes and oncogenes [5, 6]. Specific risk factors for disease progression to promyelocytic BP of CML have not been identified so far. Few studies have suggested selective suppression of the Philadelphia-positive clone by TKI and TKIinduced chromosomal aberrations [1, 3]. The longer the delay in starting TKI therapy, the more the cells exposed to genomic instability [5]. This finding suggests that PML-RARA clones may have already existed with the BCR-ABL1 clone before the initiation of TKI therapy and may have multiplied rapidly during TKI therapy, which only killed the BCR-ABL1 clone, although we could not confirm the presence of a PML-RARA clone in the sample used for primary diagnosis.
Among the reported four patients, three showed bleeding symptoms and accompanying DIC. Coagulopathy is frequently observed in APL and is associated with early death [7]. Thus, when CML patients show bleeding symptoms, early detection of disease progression and starting adequate treatment immediately are critical.
Few studies have reported APL with both PML-RARA and BCR-ABL1 rearrangements [8, 9]. If a patient is primarily diagnosed as having APL with both PML-RARA and BCR-ABL1 rearrangements, the possibility of progression of undiagnosed CML to promyelocytic BP, rather than de novo APL with both PML-RARA and BCR-ABL1 rearrangements should be considered [10].
In conclusion, disease progression of CML to promyelocytic BP should be considered when (1) BCR-ABL1 and PML-RARA rearrangements are detected simultaneously and (2) sudden changes in leukocyte counts or bleeding symptoms occur despite TKI therapy, which could be an important clue suggesting disease progression to promyelocytic BP rather than adverse effects of TKI.
Notes
REFERENCES
1. Chung HJ, Chi HS, Cho YU, Park CJ, Seo EJ, Kim KH, et al. 2008; Promyelocytic blast crisis of chronic myeloid leukemia during imatinib treatment. Ann Clin Lab Sci. 38:283–6. PMID: 18715859.
2. Oku E, Imamura R, Nagata S, Takata Y, Seki R, Otsubo K, et al. 2007; Promyelocytic crisis of chronic myelogenous leukaemia during imatinib mesylate treatment. Acta Haematol. 117:191–6. DOI: 10.1159/000097920. PMID: 17170522.

3. Hoehn D, Lu G, Konoplev S, Zhou Y, Bueso-Ramos CE, Zuo Z, et al. 2012; t(15;17)(q24.1;q21.2)/PML-RARA in blast phase of chronic myelogenous leukemia: a rare form of clonal evolution. J Hematopathol. 6:187–93. DOI: 10.1007/s12308-012-0172-6.
4. Taylor FB Jr, Toh CH, Hoots WK, Wada H, Levi M. 2001; Towards definition, clinical and laboratory criteria, and a scoring system for disseminated intravascular coagulation. Thromb Haemost. 86:1327–30. DOI: 10.1055/s-0037-1616068. PMID: 11816725.


5. Radich JP. The biology of CML blast crisis. Hematology Am Soc Hematol Educ Program. DOI: 10.1182/asheducation-2007.1.384. PMID: 18024655.

6. Saußele S, Silver RT. 2015; Management of chronic myeloid leukemia in blast crisis. Ann Hematol. 94(S2):S159–65. DOI: 10.1007/s00277-015-2324-0. PMID: 25814082.

7. Breen KA, Grimwade D, Hunt BJ. 2012; The pathogenesis and management of the coagulopathy of acute promyelocytic leukaemia. Br J Haematol. 156:24–36. DOI: 10.1111/j.1365-2141.2011.08922.x. PMID: 22050876.


8. An GD, Lim HH, Woo KS, Kim KH, Kim JM, Kim SH, et al. 2017; A case of acute promyelocytic leukemia with co-existence of BCR-ABL1 and PML-RARA rearrangements detected by PCR. Lab Med Online. 7:196–200. DOI: 10.3343/lmo.2017.7.4.196.
9. Sun X, He Y, Mao C, Zhu L, Qin X, Huang S. 2014; BCR/ABL fusion gene detected in acute promyelocytic leukemia: a case study of clinical and laboratory results. Leuk Lymphoma. 55:435–8. DOI: 10.3109/10428194.2013.800870. PMID: 23772664.


10. Chung H, Hur M, Yoon S, Hwang K, Lim H, et al. 2020; Performance evaluation of the QXDx BCR-ABL %IS droplet digital PCR assay. Ann Lab Med. 40:72–5. DOI: 10.3343/alm.2020.40.1.72. PMID: 31432643. PMCID: PMC6713652.

FIGURE AND TABLE
Fig. 1
Results of BM study, RT-qPCR, and FISH assay at the diagnosis of blast phase of CML. (A) Abnormal promyelocytes on a BM aspirate smear (Wright–Giemsa stain, × 1,000). (B) RT-qPCR analysis of BCR-ABL1 translocation (HemaVision-28N, DNA Diagnostic, Risskov, Denmark), showing a 397-bp band in the M6B split-out PCR and a 353-bp band in the M8C split-out PCR, indicating BCR-ABL1 (b3a2) and PML-RARA (bcr1 isoform) fusion transcripts. (C and D) FISH using a break-apart probe for BCR-ABL1 and PML-RARA fusion genes (Cytocell, Cytocell Ltd, Oxford Gene Technology, Cambridge, UK) showed two fusion signals, one green and one red, suggesting both BCRABL1 and PML-RARA rearrangements.
Abbreviations: BM, bone marrow; CML, chronic myeloid leukemia; FISH, fluorescence in-situ hybridization; RT-qPCR, quantitative reverse-transcription PCR.
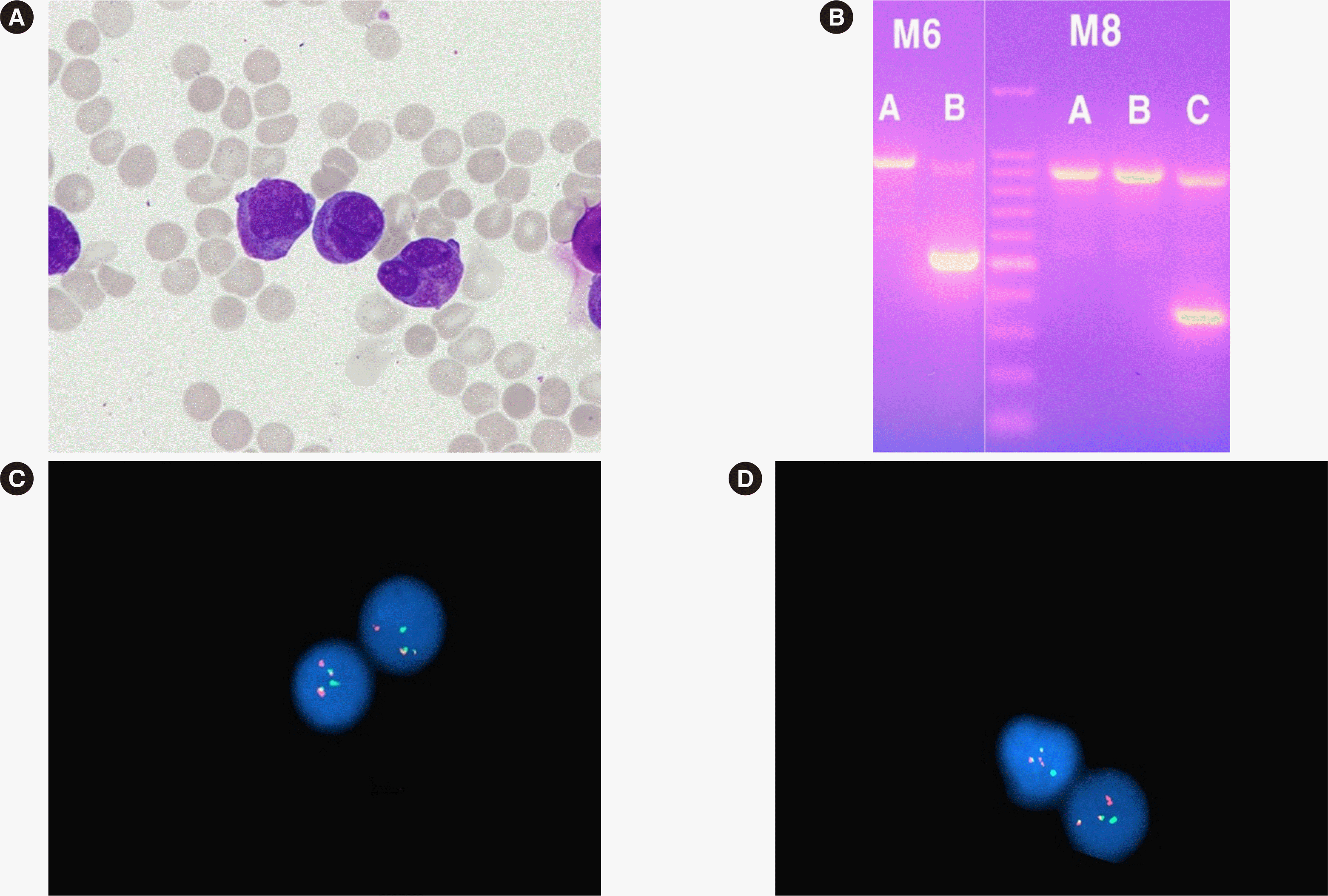
Table 1
Clinical and laboratory features of patients with promyelocytic BP of CML with both BCR-ABL1 and PML-RARA rearrangements after TKI therapy
| Oku, et al. 2007 [1] | Chung, et al. 2008 [2] | Hoehn, et al. 2013 [3] | Current study | |
|---|---|---|---|---|
| Age/gender | 66/F | 32/M | 72/F | 35/M |
| At primary diagnosis of CML, CP | ||||
| WBC count (×109/L) | 16.20 | 16.30 | 39.80 | 60.55 |
| Hemoglobin (g/L) | Not reported | Not reported | Not reported | 137 |
| Platelet count (×109/L) | Not reported | Not reported | Not reported | 413 |
| Peripheral blood finding | Not reported | 2% blasts | 1% blasts | Occasional blasts, 14% myelocytes, 12% metamyelocytes, 6% band neutrophils, 55% neutrophils |
| Splenomegaly | Not reported | Mild | Not reported | None |
| Bone marrow finding | 7.20% blasts, M:E ratio 9.55:1 | 1.00% blasts, M:E ratio 26.8:1 | Not reported | 1.20% blasts, M:E ratio 12.6:1 |
| Chromosome | 46,XX,t(9;22)(q34;q11) | 46,XY,t(9;22)(q34;q11) | 46,XX,t(9;22)(q34;q11) | 46,XY,t(9;22)(q34;q11) |
| FISH for major BCR-ABL1 rearrangement | Positive (89.3%) | Not reported | Positive | Not tested |
| Quantity of BCR-ABL1 transcript | Not reported | 0.016 | Not reported | 30.02% |
| Treatment | Imatinib 400 daily | Imatinib 400 daily | Imatinib | Dasatinib 100 mg daily two yrs after diagnosis |
| Clinical course after TKI therapy | CHR, good CCyR after eight months | CHR after three months | CCyR and MMR | Not evaluated |
| At diagnosis of CML, BP | ||||
| Time to progression to BP | 16 months after diagnosis | Six months after diagnosis | Not reported | 26 months after diagnosis |
| WBC count (×109/L) | 0.30 | 4.90 | 17.80 | 45.67 |
| Hemoglobin (g/L) | 96 | 95 | 98 | 79 |
| Platelet count (×109/L) | 49 | 15 | 9 | 3 |
| Peripheral blood finding | 91% leukemic promyelocytes | 53% leukemic promyelocytes | 90% leukemic promyelocytes | 97% leukemic promyelocytes |
| Bleeding symptom | Gingival bleeding | None | Ecchymosis | Oral bleeding |
| Prothrombin time | 47% (reference range 60–130) | 12.6 sec (reference range 10.0–13.0) | 19.0 sec (reference range 12.7–15.0) | 18.6 sec (reference range 9.5–12.3) |
| Fibrinogen (g/L) | 0.57 (reference range 2.00–4.00) | 2.09 (reference range 2.00–4 00) | 1.56 (reference range 2.02–4 50) | 0.96 (reference range 1.94–4.32) |
| D-dimer (mg/L FEU) | Not reported | 87.10 (reference range <0.40) | >20.00 (reference range <0.40) | 6.65 (reference range <0.48) |
| Presence of DIC | Yes | Yes | Yes | Yes |
| Bone marrow finding | 26.8% leukemic promyelocytes | 86.6% leukemic promyelocytes | 90.0% leukemic promyelocytes (microgranular variant) | 96.2% leukemic promyelocytes |
| Immunophenotyping | Positive: CD13 and CD33 Negative: CD34 and HLA-DR | Positive: CD13, CD33, and CD117 Negative: CD34 and HLA-DR | Positive: CD2, CD13, CD15, CD33, CD34, CD56, CD64, CD117, and MPO Negative: HLA-DR | Positive: CD13, CD15, CD33, CD64, CD117, and MPO Negative: CD34 and HLA-DR |
| Chromosome | 46,XX,t(9;22)(q34;q11.2),t(15;17)(q24;q21) | Not reported | 46,XX,der(3)t(3;15)(q21;q15)t(15;17)(q24.1;q21.2),t(9;22)(q34;q11.2),der(15)t(3;15),del(17)(q21)[20] | 46,XY,t(9;22)(q34;q11.2),t(15;17)(q24;q21) |
| FISH for major BCR-ABL1 rearrangement | 89.3% fusion signal | 96.0% fusion signal | 85.5% fusion signal | 100% fusion signal |
| FISH for PML-RARA rearrangement | 95.0% fusion signal | 90.0% fusion signal | 81.5% fusion signal | 100% fusion signal |
| Quantity of BCR-ABL1 transcript | Not reported | 2.21 | 99.73% | 53.22% |
| Quantity of PML-RARA transcript | Not reported | 0.81 | 13.28% | Not tested |
| Treatment | Idarubicin, Ara-C and ATRA | ATRA + imatinib | ATRA and arsenic trioxide | Idarubicin, ATRA and dasatinib |
| Clinical course | Normal hematopoiesis recovered | Not presented | Expired after two months | Marrow cellularity recovered, but did not reach CR |
Abbreviations: M, male; F, female; BP, blast phase; CML, chronic myeloid leukemia; WBC, white blood cells; TKI, tyrosine kinase inhibitor; FISH, fluorescence in-situ hybridization; CHR, complete hematologic response; CCyR, complete cytogenetic response; DIC, disseminated intravascular coagulation; Ara-C, cytarabine; ATRA, all-trans-retinoic acid; CR, complete response; MMR, major molecular response; CP, chronic phase; M:E, myeloid:erythroid.




 PDF
PDF Citation
Citation Print
Print



 XML Download
XML Download