Abstract
With the ongoing novel coronavirus disease 2019 (COVID-19) pandemic, the number of individuals that need to be tested for COVID-19 has been rapidly increasing. A walk-through (WT) screening center using negative pressure booths that is inspired by the biosafety cabinet has been designed and implemented in Korea for easy screening of COVID-19 and for safe and efficient consultation for patients with fever or respiratory symptoms. Here, we present the overall concept, advantages, and limitations of the COVID-19 WT screening center. The WT center increases patient access to the screening clinics and adequately protects healthcare personnel while reducing the consumption of personal protective equipment. It can also increase the number of people tested by 9–10 fold. However, there is a risk of cross-infection at each stage of screening treatment, including the booths, and adverse reactions with disinfection of the booths. These limitations can be overcome using mobile technology and increasing the number of booths to reduce congestion inside the center, reducing booth volume for sufficient and rapid ventilation, and using an effective, harmless, and certified environmental disinfectant. A WT center can be implemented in other institutions and countries and modified depending on local needs to cope with the COVID-19 pandemic.
Graphical Abstract
The novel coronavirus disease (COVID-19) has been spreading globally since its outbreak was announced in December 2019. It is caused by severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2). On March 12, 2020, the World Health Organization (WHO) declared a pandemic of COVID-19, and as of March 16, 2020, more than 160,000 confirmed cases have been reported in 151 countries.1
In such a pandemic situation, it is important to protect medical staffs and use resources such as personal protective equipment (PPE) safely and efficiently. For this purpose, a drive-through (DT) screening center was developed and implemented in Korea and introduced in other countries as well.2 However, there are many medical institutions that find it difficult to install or operate a DT screening center and there are many patients who find it difficult to visit it in their own cars. Herein, we introduce a walk-through (WT) screening center for COVID-19 and share our experience with healthcare authorities and providers globally.
Based on the Safe Assessment and Fast Evaluation Technical booth of the H Plus Yangji Hospital (SAFETY), a WT screening center was designed and implemented at H Plus Yangji Hospital, Seoul. This general hospital with 350 beds is located in Gwanak-gu, one of the busiest places in Seoul. As of 2018, the total population of Gwanak-gu was approximately 500,000. From 2015 to the date of writing, this hospital has been the only regional emergency center in Gwanak-gu.3 When this hospital started a screening clinic, there was only one other screening clinic in Gwanak-gu. Around 50–80 people visited the screening clinic during the operating hours. The hospital space, including the parking space, was not large enough to install a DT screening center or a well-ventilated outdoor screening center. In addition, because of the regional nature of Gwanak-gu, there was a large proportion of patients who need to visit the screening clinic on foot.
As a standard method, the following procedure should be performed before examining the next patient. First, the healthcare personnel (HCP) should properly doff the PPEs and don new PPEs. Second, the test site should be ventilated so that the SARS-CoV-2 remains less than 1% in the air. Third, the environment of the test site should be disinfected properly. Finally, it should be sufficiently ventilated until the disinfectant dries and the residual effect of the disinfectant disappears. It took more than 30 minutes to complete all of these steps. Although the only HCP trained to properly use PPE participated in screening process, only 9–10 people could be tested per day. Moreover, in this COVID-19 outbreak, there were not enough PPE resources. In this situation, it was difficult to follow the standard process accurately. As a result, the infection risk among patients as well as HCP had increased. For these reasons, SAFETY and the SAFETY-based WT center were designed.
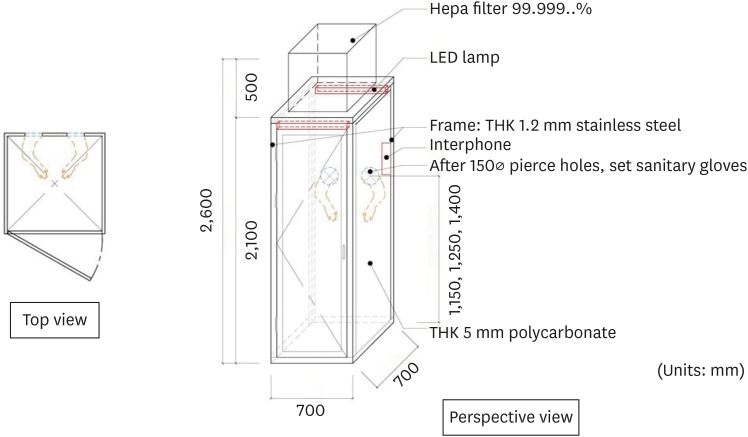
SAFETY is a negative pressure booth for one person, inspired by the Biosafety Cabinet used in the bio lab. A schematic drawing of the SAFETY is given in Fig. 1. SAFETY has an area of 0.49 m2 and a height of 2.05 m; each side of SAFETY is made of a polycarbonate plate on a stainless steel frame. The WT center has 4 SAFETYs. For each SAFETY, the internal negative pressure was maintained using a mobile negative pressure device with a maximum air volume of 1,000 m3/hour and a high-efficiency particulate air filter. The estimated ventilation rate was 100 air change/hour. Around 3 and 5 minutes were required to remove 99% and 99.9% of the particles from the air, respectively.4 In the direction of the medical staff, a glove wall was installed with the glove facing towards the inside of the SAFETY. The height of the glove entrance was different for each SAFETY, thereby allowing examination according to the patient's height (Fig. 2). A stethoscope was installed on the glove wall. The interphone was installed inside the SAFETY so that the patient could communicate smoothly with the medical staff outside the booth. Sample collection kit, disposable tongue depressor, and medical pen light were provided. The patient's entrance was opposite the glove wall. The total cost to build a booth was approximately 2,400 dollars.
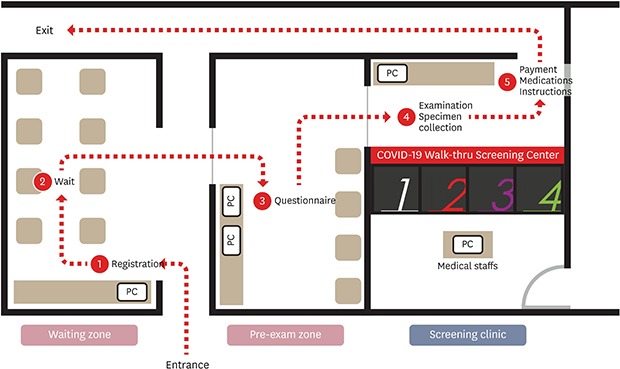
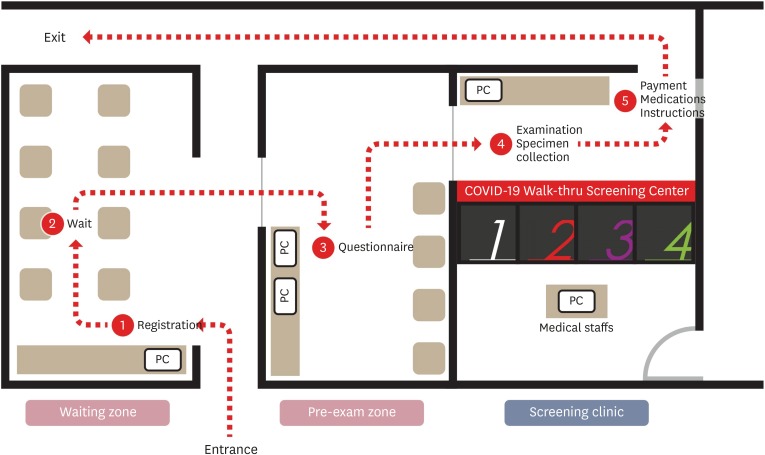
The brief flow of the WT center was as follows: Entrance – Registration – Wait – Questionnaire – Examination and Specimen Collection – Medication and Instructions – Exit (Fig. 3). From the entrance to the exit, all patients were asked to wear a mask and move under the guidance of the HCP. At the entrance of the waiting zone, there was a tablet personal computer (PC) for registration and chairs were placed inside the waiting zone to allow waiting patients to sit > 2 m apart. When a patient entered his/her mobile phone number on the tablet PC and registers, a waiting number and entry request message was sent by a text or SNS to enter the waiting zone without waiting in line. While the patient was waiting for the waiting zone entry sequence, a mobile questionnaire was prepared in advance using a QR code. If the patient was unable to complete the mobile questionnaire, he/she entered the pre-exam zone and filled out an electronic questionnaire using a computer. The pre-exam zone had 2 laptop computers, which were disinfected after each patient's use and alternately used while the disinfectant dried. Upon completion of the electronic questionnaire or mobile questionnaire, this form was delivered to the medical staff through the hospital information system (HIS). The doctor checked the questionnaire and allowed the patient to enter the assigned booth if a medical examination or sample collection was necessary. After the patient entered the booth, the medical staff on the other side conducted an interview through the interphone and conducted examination and sample collection through the glove wall. Upon completion of the sample collection, the patient wore a mask, exited the booth, stored it, received medicine and instructions, and returned home. After the patient left the booth, ventilation was performed for an appropriate period of time, environmental disinfection was performed using an appropriate environmental disinfectant, ventilation was again performed after disinfection completed before the next patient entered the room. The ventilation time for each safety was checked using a timer and the booth under disinfection and the booth after disinfection were marked with a sign. The outer gloves were applied to the gloves inside the booth to be replaced for each patient. After the introduction of the WT screening center, the average number of patients that could be tested in 8 hours increased from 10 to > 70.
The most important process in the WT center is environmental cleaning. Environmental cleaning is performed in the following order: ventilation after the patient has left, disinfection of the surface of the booth including gloves and ventilation after disinfection. An important part of surface disinfection is disinfecting gloves, maintaining sufficient contact time according to the disinfectant manufacturer's instructions and ventilation after disinfection according to the residual toxicity of the disinfectant.
The booth door was closed after the patient left and ventilated for 5 minutes. After ventilation is complete, the outer gloves were removed and the inside surface of the booth, including the inner gloves, were disinfected. The outer gloves were replaced for each patient, and the inner gloves were also replaced if abnormalities were found. The ventilation time after disinfection depended on the type of the disinfectant. The Centers for Disease Control and Prevention (CDC),5 World Health Organization (WHO),6 and Korea Centers for Disease Control and Prevention (KCDC)78 guidelines recommend that the ventilation time after disinfection should be set according to the manufacturer's instructions for each disinfectant.
When the WT center of the H Plus Yangji Hospital started operating, the booth surface was sterilized by spraying with a disinfectant containing quaternary ammonium compounds. However, this method of spraying the disinfectant was discontinued because it was not recommended in the guidelines. Instead, the surface was wiped with mops. Even though the disinfectant was ventilated until it was completely dry after disinfection, it caused eye irritation in the hospital staff who entered the booth for work. Therefore, the disinfectant was changed to 1,000 ppm sodium hypochlorite. Sodium hypochlorite also caused skin irritation. In addition to the above two components, the components of surface disinfectants recommended by the CDC, WHO, or KCDC are alcohol (ethyl or isopropyl) and enhanced hydrogen peroxide. Of these two components, alcohol is not recommended to disinfect large environmental surfaces. Hence, we had to find a disinfectant containing enhanced hydrogen peroxide. The CDC guidelines recommend applying a United States Environmental Protection Agency (EPA)-registered disinfectant against SARS-CoV-2 for environmental cleaning and disinfection in healthcare settings, including those patient-care areas where aerosol-generating procedures were performed.5 Oxivir® Tb solution, an enhanced hydrogen peroxide disinfectant, is one of the EPA-registered disinfectants.9 Also, the KCDC guidelines recommend the use of Oxivir® Tb solution, too.78 This disinfectant was made with 0.5% enhanced action formulation of hydrogen peroxide. This disinfectant can kill enveloped viruses such as SARS-CoV-1, non-enveloped viruses and bacteria with a contact time of 1 minute. In addition, after disinfecting, the disinfectant decomposes into water and oxygen and is harmless to the environment and the human body.10 Theoretically, it is sufficient to ventilate for 5 minutes after disinfecting the surface to maintain a contact time of 1 minute or longer with this disinfectant. However, there were 4 booths in the WT center, and it took about 30 minutes for the next patient to enter the same booth after disinfecting one booth. For evaluating environmental cleaning after daily screening clinic work, environmental samples were collected and tested using the polymerase chain reaction (PCR) method in the area where the patient's face was located in the booth. As of March 31, 2020, two COVID-19 confirmed patients were screened at our screening clinic, but the results of the daily environmental PCR tests were all confirmed negative.
In the current pandemic situation, a WT screening center using SAFETY has increased accessibility and efficiency. It can effectively reduce the consumption of limited PPE resources and reduce the fatigue and risk of infection associated with frequent PPE replacement. Also, as compared to a DT screening center, it is easy to apply in situations where patients visit on foot in a relatively small space.2
By reducing the volume of one booth, the ventilation rate is increased and the time required for ventilation and surface disinfection is reduced. Several booths can be installed in a limited space. Even if four booths are installed, it is possible to examine 9–10 times more patients in the same time than that using the conventional method. If more booths are installed and the diagnostic capacity of the laboratory is increased, more patients can be tested in the same time. It also helps lower the congestion of patients in the WT center by increasing the speed of the test. The safe and efficient use of these resources allows continued and appropriate screening care.
The most important issue with the WT screening center is the disinfection of booths. The goal is to disinfect the surface with an appropriate disinfectant with sufficient time of ventilation after the patient has left and allow the next patient to safely enter the room after the disinfectant has sufficiently dried and the booth is ventilated. Surface disinfection should be done with minimal consumption of PPE. Selecting an appropriate and safe disinfectant is the most important issue.
Reducing patient congregation and the number of staffs in direct contact with the patient during all processes at the WT center is also important. To achieve this, we applied a mobile waiting system with SNS or text messages, electronic questionnaires which were connected to the HIS and unmanned payment machines. Patients can fill out and send the electronic questionnaires to HIS by their own mobile phones from anywhere outside the WT center.
COVID-19 can cause severe pneumonia. It is important to understand patient's severity of illness. SAFETY allows the doctor to directly see and examine the patient in the booth with a stethoscope. Safely assessing oxygen saturation, blood pressure, and chest radiography will further help assess the severity.
A screening system using a well-ventilated open space may be considered. In this case, the cost for initial installation and maintenance may be less than that of H Plus Yangji Hospital's WT system. However, because natural ventilation is influenced by wind speed, direction, temperature and humidity, natural ventilation may not be performed properly. In addition, natural ventilation precludes the use of particulate filters.11 For this reason, installation requires a large space with little human traffic and good wind. Also, the number of patients that can be tested in the same space is limited. It is difficult to apply this system when it is difficult to secure an open space for screening, such as H Plus Yangji Hospital. In the case of DT center, it is possible to consider using a single N95 respirator for less than 4 hours because respiratory droplets may be blocked by the window of the car during sample collection.2 However, when the WT center is installed outdoors and samples are collected by conventional methods, it is necessary to properly replace the PPE for each patient in principle because HCP are more directly exposed to the patient's respiratory droplets. And in this case, PPE consumption may increase. If PPE resources are limited, the risk of HCP exposure to infection may increase. Another option is to take the booth glove-wall outwards so that the medical staff can enter the booth and take a patient's sample. In this case, as with the WT center of H Plus Yangji Hospital, proper environmental disinfection needs to be considered after sampling. The CDC guideline recommends that specimens such as nasopharyngeal swabs are closed in the examination room.5
If an ideal booth with all the functions of the WT center is built and well connected to the public healthcare system, these booths might be installed in public places such as airports and train stations as well as hospitals like public telephone boxes. For example, a telemedicine system might be used to allow a patient to receive a doctor's treatment, including sample collection or chest X-ray examination, in a booth. The electronic payment system is used to make payments, and electronic prescriptions can be issued to receive medicines through an appropriate delivery system. The inside of the booth is automatically cleaned with a suitable disinfectant. If the patient receiving medical treatment at the booth needs to be transported to the hospital, an ambulance and emergency transport team come to the booth to transport the patient. In addition to COVID-19, such system might be used to cope with infectious fever and respiratory diseases that may occur in the future.
In the COVID-19 pandemic era, a WT screening center enables safe and efficient treatment while maintaining accessibility to the healthcare system even in resource-limited situations. We hope to be the cornerstone in developing a WT system with the least patient contact through appropriate infection control and modification using current and future technologies.
References
1. World Health Organization. Coronavirus disease 2019 (COVID-19) Situation Report – 56. Updated 2020. Accessed March 16, 2020. https://www.who.int/docs/default-source/coronaviruse/situation-reports/20200316-sitrep-56-covid-19.pdf?sfvrsn=9fda7db2_6.
2. Kwon KT, Ko JH, Shin H, Sung M, Kim JY. Drive-through screening center for COVID-19: a safe and efficient screening system against massive community outbreak. J Korean Med Sci. 2020; 35(11):e123. PMID: 32193904.

3. Korea Statistics. Population census. Updated 2020. Accessed April 2, 2020. http://kosis.kr/statisticsList/statisticsListIndex.do?menuId=M_01_01&vwcd=MT_ZTITLE&parmTabId=M_01_01#SelectStatsBoxDiv.
4. Jensen PA, Lambert LA, Iademarco MF, Ridzon R; CDC. Guidelines for preventing the transmission of Mycobacterium tuberculosis in health-care settings, 2005. MMWR Recomm Rep. 2005; 54(RR-17):1–141.
5. Centers for Disease Control and Prevention. Interim infection prevention and control recommendations for patients with suspected or confirmed coronavirus disease 2019 (COVID-19) in healthcare settings. Updated 2020. Accessed March 23, 2020. https://www.cdc.gov/coronavirus/2019-ncov/infection-control/control-recommendations.html.
6. World Health Organization. Infection prevention and control during health care when novel coronavirus (nCoV) infection is suspected. Interim guidance. Updated 2020. Accessed March 25, 2020. https://www.who.int/publications-detail/infection-prevention-and-control-during-health-care-when-novel-coronavirus-(ncov)-infection-is-suspected-20200125.
7. Centers for Disease Control and Prevention. Updated 2020. Accessed March 20, 2020. http://ncov.mohw.go.kr/searchBoardView.do?brdId=2&brdGubun=25&dataGubun=&ncvContSeq=1085.
8. Centers for Disease Control and Prevention. Updated 2020. Accessed March 25, 2020. https://www.cdc.go.kr/board/board.es?mid=a20507020000&bid=0019.
9. United states Environmental Protection Agency. List N: disinfectants for use against SARS-CoV-2. Updated 2020. Accessed March 24, 2020. https://www.epa.gov/pesticide-registration/list-n-disinfectants-use-against-sars-cov-2.
10. Oxivir® Tb RTU. Accessed March 22, 2020. http://solutionsdesignedforhealthcare.com/solutions/products/disinfectants/oxivir-tb-rtu.
11. Chartier Y, Pessoa-Silva C. Natural Ventilation for Infection Control in Health-care Settings. Geneva: World Health Organization;2009.




 PDF
PDF Citation
Citation Print
Print




 XML Download
XML Download