Abstract
Acute pancreatitis (AP) severity is determined by associated organ failure (OF). However, enzymatic erosion of peripancreatic vessels can lead to life-threatening hemoperitoneum in clinically non-severe AP without OF. We herein report a case of unexpected hemoperitoneum which developed in a patient with clinically resolving AP without OF. A 36-year-old woman with alcohol use disorder presented with resolving epigastric pain and sustained abdominal distension of 2 weeks’ duration. Ranson’s score on admission was 1 and Computed tomography (CT) revealed non-necrotic AP with peripancreatic fluid collection. She showed sudden hypotension with an abrupt decrease in serum hemoglobin within 24 hours after admission. She was suspected to have an acute hemoperitoneum associated with venous bleeding from AP based on repeated CT. Venous bleeding from the splenic branch was ligated during surgery. The possibility of bleeding at the pancreatic bed should be considered even if the pancreatitis is not severe.
Anatomic complications develop only in 20% of acute pancreatitis (AP) cases.1 The overall mortality associated with AP is less than 5%.1 AP severity is determined by associated organ failure (OF).2 However, enzymatic erosion of peripancreatic vessels can lead to life-threatening hemoperitoneum in both clinically non-severe and resolving AP even without OF. We herein report a case of unexpected hemoperitoneum that developed in a patient with clinically resolving AP without OF and was successfully treated using surgical ligation.
A 36-year-old woman with alcohol use disorder presented at the outpatient clinic with improving epigastric pain and sustained abdominal distension of 2 weeks’ duration. She previously had several episodes of acute epigastric pain radiating to the back, which persisted for several hours, during two months and improved. She only had abdominal distension during the first visit, which makes deep breathing uncomfortable. She was previously diagnosed with hypertension, dyslipidemia, and polycystic kidney disease. She was taking bisoprolol and fenofibrate. Her history showed alcohol consumption of 1 bottle per day, 4 times a week, for 10 years. Her vital signs were stable with blood pressure 138/97 mmHg, heart rate 73 beats/minute, respiration rate 18 beats/minute, and body temperature 36.6°C. She did not appear to be acutely ill upon physical examination. Conjunctiva were not pale and sclera were anicteric. There was periumbilical tenderness and shifting dullness without abdominal guarding. Laboratory results revealed hemoglobin level 11.8 g/dL (hematocrit 35.3%), white blood cells count (WBC) 6,260/uL, and platelet count 211,000/uL. Prothrombin time (international normalized ratio, INR) was 1.39. Serum total protein and albumin levels were 4.8 and 2.6 g/dL, respectively, and aspartate aminotransferase and alanine aminotransferase levels were 55 and 13 IU/L, respectively. Alkaline phosphatase and gamma glutamyl transferase levels were 58 and 62 U/L, respectively, and total bilirubin level was 0.7 mg/dL. Serum amylase and lipase levels were 216 (normal, 22 – 80) and 565 (normal, 0 – 67) U/L, respectively, which revealed that the recent AP seemed to be resolving. Serum lactic dehydrogenase (LDH) level was 523 U/L and triglyceride was 113 mg/dL. Chest and abdominal x-ray scans were normal. Liver dynamic computed tomography (CT) was used to evaluate the possibility of chronic liver disease or pancreatitis related with chronic alcohol use 6 hours after admission. The liver surface and contour were unremarkable. However, there were multiple small cystic lesions in the pancreas with peripancreatic fat infiltration and fluid collection loculated at the head portion (Fig. 1). Additionally, there was a moderate amount of ascites. Diagnostic paracentesis was performed with ultrasound assistance at the right lower quadrant area. The ascitic fluid had a bloody appearance and serum ascites albumin gradient was 0.7. In ascitic analysis, total protein level 3.2 g/dL, albumin level 1.9 g/dL, LDH level 314 U/L, glucose level 139 mg/dL, red blood cell count 1,400,000/uL, and WBC count 1850/uL (neutrophil 13% and monocyte 72%) were found. We did not suspect hemoperitoneum because CT scan did not reveal any blood at initial evaluation and her vital signs were stable. She complained of dizziness and nausea on the second day of admission, 20 hours after the first CT scan. Her heart rate increased to 125 beats/minute without hypotension, 29 hours after the initial CT scan. Laboratory findings revealed that hemoglobin dropped to 6.9 g/dL from 11.8 g/dL. Serum amylase and lipase were 94 and 222 U/L. Follow-up CT scan revealed a newly developed hemoperitoneum in the left upper quadrant, left paracolic gutter, and pelvic cavity without active contrast media extravasation. After re-evaluation of the initial CT scan, undetected active leakage of contrast media from the mesenteric vessel at the level of duodenojejunal junction in the venous phase was found (Fig. 2). General surgery physicians had been closely monitoring the vital signs and decided to delay the time point for the exploratory laparotomy until the activity of internal bleeding is confirmed on radiologic findings. On the third day and sixth day of admission, there were 2 events of hypotension (74/53 mmHg) and tachycardia (125 beats/minute) with less than 2 g/dL decrease of hemoglobin. Six units of packed red blood cells (PRBC) were used to compensate for continuous venous bleeding during 5 days of observation without significant collapse until laparoscopic evaluation. Finally on the seventh day, we conducted the surgery on the patient. 2 L of blood from the hematoma were evacuated in the operation room, but the focus of active bleeding was not found and the suspected focus visible on CT scan could not be ligated. Four more units of PRBC were transfused. After the first operation, about 1 L of fresh blood was consistently drained through hemovac during the first 24 hours with a single event of hypotension (76/51 mmHg) and tachycardia (121 beats/minute), which led to a second emergency operation. During the operation, there was venous bleeding near the lateral border of the Treitz ligament from the splenic branch on the lower margin of the pancreas. The bleeding was controlled using surgical ligation. Further bleeding was not observed after the second operation and she was discharged 10 days later.
AP is an acute inflammatory process in the pancreas with or without the involvement of regional tissues or distant organs.1 Disease severity is classified based on the presence of local/systemic complications and/or OF.2 Without OF, severe AP is excluded by definition and in-hospital mortality of AP without OF is significantly lower than that of AP with any OF (2% vs. 28%).3 Therefore, local complication itself is not the determinant in defining the severity.2 However, acute intraabdominal hemorrhage, a rare type of local complication, is associated with high mortality rates similar to severe AP.2,4
We herein report a case of an unexpected hemoperitoneum which developed in a patient with clinically resolving AP without OF. At the time of diagnosis, epigastric pain which developed 2 months ago was improved and decreased amylase and elevated lipase levels revealed resolving pancreatitis. Although the CT scan revealed a Balthazar grade D AP without necrosis, Ranson’s score was 1 on admission and 2 after 48 hours of admission (LDH level 523 U/L and 14% decrease of hematocrit). She could have been observed at an outpatient clinic because her symptoms were relieving. However, fatal hemoperitoneum developed within 24 hours of presentation and 10 units of PRBCs were transfused to sustain and stabilize her vital signs until the bleeding focus could be found and surgically ligated.
Acute bleeding into the gastrointestinal (GI) tract or intraabdominal cavity can develop from severe inflammation and liberated activated enzymes, which erode the local vessels during AP.1,4 Ruptures of the splenic artery, splenic vein, and portal vein are frequently reported.4 Other mechanisms include rupture of pseudoaneurysm of the splenic artery, pressure necrosis of vascular structure by pseudocysts, or pancreatic abscess. 1 Several large data pools exist regarding hemorrhagic complications in AP. However, it is confusing as the data also include GI bleeding, which was either pancreatitis- or non-pancreatitis related.4–6 Recent data from a single-center study reported that the incidence of AP with hemorrhage into the pancreatic bed was 2.7%.6 Mortality rate from intra-abdominal hemorrhage was reported to be 67%.5 Diffuse peripancreatic bleeding occurred in both severe and mild forms of AP.5
We speculate that this is the first report of AP complicated by life-threatening hemoperitoneum without the presence of pancreatic necrosis or pseudoaneurysm. There was a case report of intra-abdominal bleeding from AP initially diagnosed with a Balthazar score of 2 and a Ranson’s score of 2, which led to sudden cardiac death within 22 hours after admission.7 The origin of bleeding was not identified either through CT or angiography. In contrast to this, we were able to find the focus of bleeding using a timely taken CT scan, retrospectively. In addition, continuous intraperitoneal bleeding could successfully be ligated during an adequately timed surgery. There were several events of hypotension and tachycardia with marginal hemoglobin decreasing less than 2 g/dL, but it was difficult to decide when to proceed with surgery because those events were easily restored through intravenous hydration and PRBC transfusion. The first operation was conducted after 24 hours of tachycardia and the second operation was done immediately after the development of hypotension. To summarize, to reduce the failure of the surgery, it would be important to wait and see until the active bleeding occurs in the setting of pancreatic bed bleeding due to acute pancreatitis.
In conclusion, clinically non-severe (based on revised Atlanta classification), resolving AP can be complicated by a life-threatening hemoperitoneum. Even if there is no evidence of pancreatic necrosis or pseudonaeurysm of local vascular structures, the possibility of bleeding at the pancreatic bed should be kept in mind. Venous bleeding can be surgically treated at the same time as acute active bleeding.
REFERENCES
1. Scott Tenner WMS. Acute pancreatitis. Mark Feldman LSF, Brandt Lawrence J, editors. Sleisenger and Fordtran’s Gastrointestinal and liver disease:Pathophysiology/Diagnosis/Management. 10th ed. Saunders: Elsevier;2016. p. 969–93.
2. Banks PA, Bollen TL, Dervenis C, Gooszen HG, Johnson CD, Sarr MG, et al. Classification of acute pancreatitis—2012: revision of the Atlanta classification and definitions by international consensus. Gut. 2013; 62:102–11.

3. Vege SS, Gardner TB, Chari ST, Munukuti P, Pearson RK, Clain JE, et al. Low mortality and high morbidity in severe acute pancreatitis without organ failure: a case for revising the Atlanta classification to include “moderately severe acute pancreatitis”. Am J Gastroenterol. 2009; 104:710–5.

4. Flati G, Andrén-Sandberg A, La Pinta M, Porowska B, Carboni M. Potentially fatal bleeding in acute pancreatitis: pathophysiology, prevention, and treatment. Pancreas. 2003; 26:8–14.

5. Andersson E, Ansari D, Andersson R. Major haemorrhagic complications of acute pancreatitis. Br J Surg. 2010; 97:1379–84.

6. Sharma PK, Madan K, Garg PK. Hemorrhage in acute pancreatitis: should gastrointestinal bleeding be considered an organ failure? Pancreas. 2008; 36:141–5.
Fig. 1
Liver dynamic computed tomography (CT) scan of patient taken within 4 hours of admission.
(A) There are multiple cystic lesions in pancreas presumed to be small pseudocysts in portal phase.
(B) Peripancreatic fluid collection is notable at the pancreas head in portal phase (arrow).
(C) There is active leakage of contrast media from the mesenteric vessel at duodenojejunal junction in delayed phase (arrow).
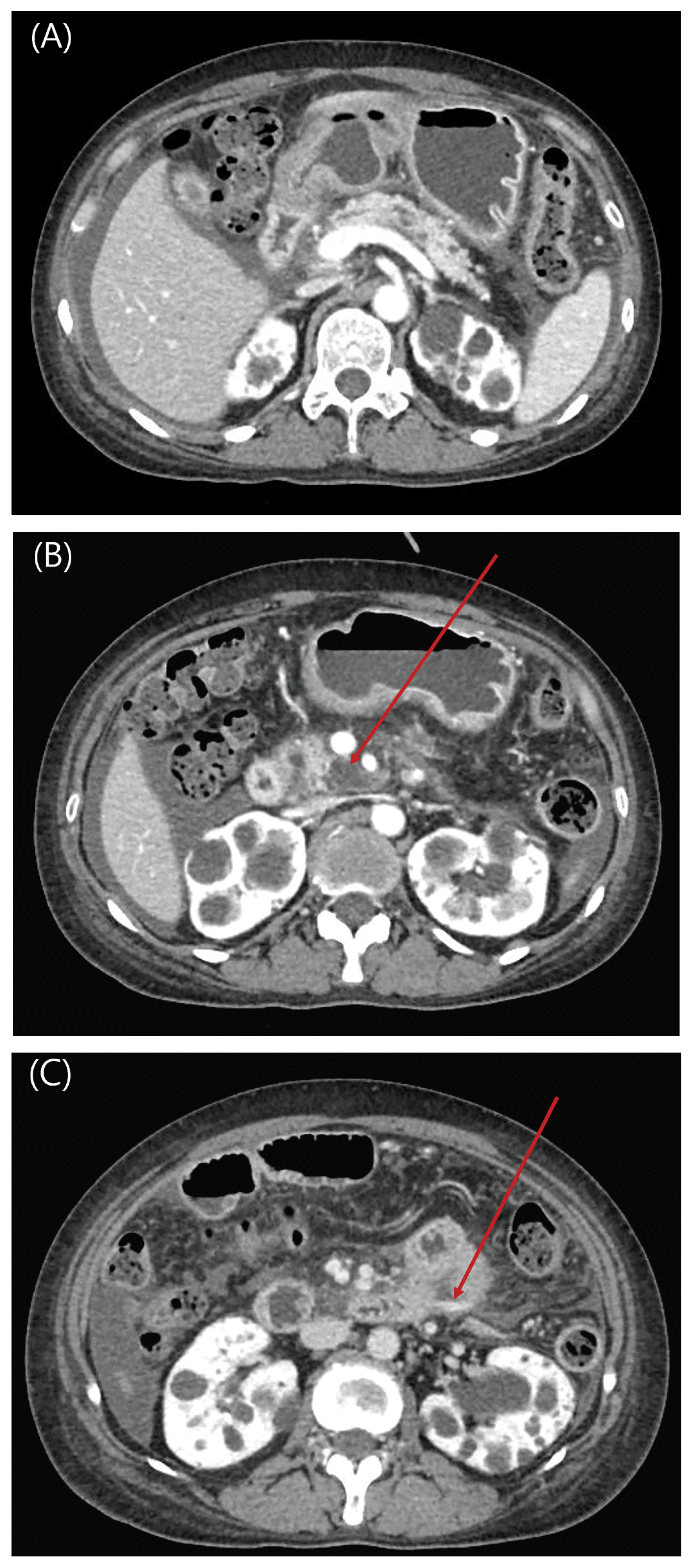
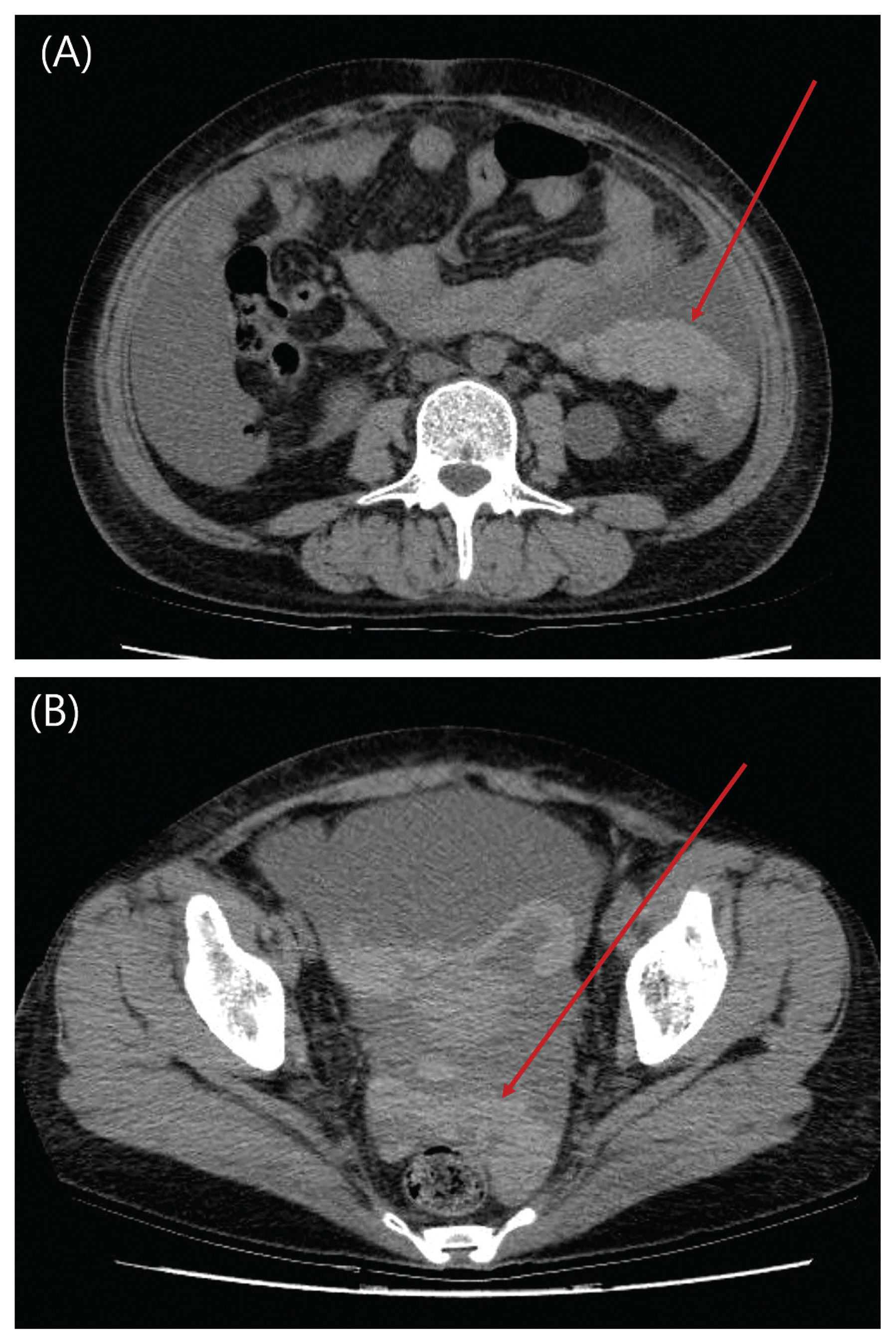
Fig. 2
Abdominal pelvis CT scan of patient taken after 29 hours of first CT scan immediately after clinical hypotensive event.
(A) There is evidence of hemoperitoneum in left upper quadrant in non-contrast enhanced image (arrow) without active leakage of contrast media in arterial phase.
(B) Newly developed hemoperitoneum is notable in pelvic cavity in non-contrast enhanced image (arrow).




 PDF
PDF Citation
Citation Print
Print


 XML Download
XML Download