Abstract
Infectious intracranial aneurysm (IIA), a rare type of cerebral aneurysm, is often observed in patients with infective endocarditis. Hemorrhage or infarction often occurs; however, the presentation of both hemorrhagic and ischemic components is rare.
A 41-year-old man with progressive motor weakness, dysarthria, and severe headache was admitted to our hospital. Brain computed tomography scan revealed a scanty subarachnoid hemorrhage (SAH), and diffusion magnetic resonance imaging confirmed acute cerebral infarction around the external capsule and insular lobe. A digital subtraction cerebral angiogram revealed an obstruction in the middle cerebral artery (MCA). The patient’s neurological symptoms improved remarkably on the fifth day, and a follow-up angiogram revealed recanalized MCA with pseudoaneurysm, which was not observed on the previous angiogram. A blood culture result confirmed bacteremia, and the patient was then diagnosed with infective endocarditis. The pseudoaneurysm was treated with anastomosis of the superficial temporal artery and MCA with trapping of the parent artery. He was discharged with no neurological deficits. Herein, we present a patient with IIA, who sequentially developed SAH and cerebral infarction, and underwent extracranial-intracranial bypass with trapping of the parent artery. Although the treatment strategy for IIA is controversial, the treatment plan should be cautiously discussed with the patient. In addition, the assessment of an underlying infectious disease is required.
Infectious intracranial aneurysm (IIA) is a rare type of cerebral aneurysm; approximately <10% of patients with bacterial endocarditis present with such condition.10) It usually causes pseudoaneurysms that result in cerebral hemorrhage or dissection, which can then lead to cerebral ischemia. Determining the best surgical, endovascular, and even medical treatments for this condition is controversial.2)3)5)7)8) Furthermore, if a patient presents with both hemorrhagic and ischemic components at the same time, the treatment becomes challenging. Herein, we present an IIA patient, who sequentially developed subarachnoid hemorrhage (SAH) and cerebral infarction, and underwent extracranial-intracranial bypass with trapping of the parent artery.
This case report was approved by the institutional review board of our hospital. Due to the retrospective design of the study, consent was neither required by the IRB nor by the study team. The informed patient consent was waived by the IRB of our institute Dankook University Hospital. IRB file 2019-05-001.
A 41-year-old man with progressive motor weakness on the right upper extremity and slurred speech, which started one day before admission, and the severe headache was admitted to our hospital. He had mild chills without fever upon arrival at the emergency room. His blood and serum analysis results showed a slightly elevated white blood cell count at 13,000/mm3 and an elevated C-reactive protein level at 3.36 mg/L. He claimed that he also had a sudden severe headache 15 days prior to admission. His past medical history was unremarkable. Brain computed tomography scan revealed a scanty SAH in the left Sylvian cistern and focal low density around the external capsule (Fig. 1). The left internal carotid artery angiogram showed an obstruction in the middle cerebral artery (MCA, a frontal branch of M2) (Fig. 2). He then developed a high fever in the intensive care unit.
Diffusion magnetic resonance imaging (MRI) confirmed acute cerebral infarction around the external capsule and insular lobe (Fig. 3A, B). As the patient had both cerebral hemorrhage and infarction, we assumed the M2 occlusion was caused by arterial dissection. Therefore, we decided to treat conservatively for the lesion. Antiplatelets or anticoagulants were not used due to the risk of bleeding in the diseased vessel. However, his motor weakness and dysarthria gradually progressed on the third day of admission. Since the extent of infarction did not improve based on follow-up brain MRI, we decided to treat him conservatively. Interestingly, the aggravated neurologic symptoms improved remarkably on the fifth day of admission.
We received the patient’s blood culture results on the fifth day of admission, and Methicillin-sensitive Streptococcus Viridans was cultured from the venous blood sample. Therefore, antibiotics were administrated according to the antibiotic sensitivity test result. In addition, rheumatic moderate mitral regurgitation, with vegetation on the mitral valve, was confirmed on transthoracic echocardiography, and the patient was diagnosed with infective endocarditis after consultation at the cardiology department (Fig. 4).
A brain MR angiography, with vessel wall imaging, showed recanalization of the frontal M2 with a fusiform aneurysm on the thirteenth day of admission (Fig. 5A). Follow-up digital subtraction cerebral angiography showed the recanalization of the frontal M2 with a fusiform aneurysm (Fig. 5B). We considered the lesion to be IIA caused by infective endocarditis. We performed surgical trapping of the IIA after anastomosis of the frontal branch of the superficial temporal artery (STA) and MCA (M4). Yellowish, inflammatory change in the arachnoid membrane was grossly observed around the left Sylvian vein (Fig. 6). The aneurysm was enveloped with inflammatory tissues, such as granuloma with severe adhesion, which made excision without injuring the normal brain parenchyma impossible. Therefore, the trapping of the parent artery using a clip was performed (Fig. 7).
Postoperative cerebral angiography confirmed the successful anastomosis of STA-MCA bypass, with adequate contrast filling, on the parietal lobe territories without visualization of the aneurysm sac (Fig. 7A, B). His clinical course had been uneventful after surgery, and he was transferred to the cardiology department for the treatment of infective endocarditis. The patient was discharged with no neurological deficits.
Baker has described four phases in the development of bacterial aneurysms.1) The infective emboli are formed, and they settle in the parent artery (phase I). This leads to the formation of an aneurysm (phase II). Although aneurysm rupture (phase III) does not occur in every case, thrombosis and spontaneous healing of the parent artery follows (phase IV). However, due to rapid progression from phase I to II, radiologic detection of intra-arterial thrombi, which leads to the formation of aneurysms, is seldom reported.11) In the present case, we assumed there was a dissection of the parent artery with/without the formation of the IIA, and this caused the severe headache the patient experienced two weeks before the presentation of neurologic symptoms. Then, a tear in the adventitia occurred shortly before the presentation of the initial neurologic symptoms, which caused SAH and activated acute thrombosis, resulting in the MCA occlusion. Therefore, the IIA was concealed in the initial angiographic studies due to the MCA occlusion, which led to progressive neurologic symptoms, including aphasia and motor weakness.
As the patient had both hemorrhagic and ischemic stroke with no evidence of pseudoaneurysm, we assumed the patient presented with dissecting aneurysm. We thought that dissection of unknown origin on the vessel caused cerebral hemorrhage, followed by an MCA occlusion, upon admission. Therefore, we decided not to use antiplatelets or anticoagulants due to the risk of re-bleeding. Even in patients with IIA, using antiplatelets or anticoagulants are not well established in previous studies.2)5)7)8) Because IIA usually forms pseudoaneurysm, which is at risk of rupture, the main focus of these studies was the use of anti-microbial therapy with/without surgical or endovascular treatment. Therefore, whether antiplatelets or anticoagulants should be used in IIA with infarction is not fully elucidated. In the present case, owing to the dynamic change in pathophysiologic conditions in the affected vessel, the clinical decision was limited, as the patient is in between presenting with hemorrhagic and ischemic conditions. The aggravation and recovery of the neurologic symptoms indicate that there was enough collateral blood supply to prevent permanent brain damage. In addition, endovascular trapping of the parent vessel without bypass surgery is not a suitable treatment option for this patient. Therefore, to avoid neurological damage in the case of a second ischemic attack, we performed STA-M4 end-to-side bypass with surgical trapping of the aneurysm.
To the best of our knowledge, there were only four reported cases of IIA with subsequent development of both cerebral hemorrhage and infarction.4)6)9)12) In all cases, cerebral hemorrhage was observed after infarction in the brain (Table 1). Moreover, the infarction, initiated by the infective embolus, occluded the parent vessel and formed dissection or pseudoaneurysm due to the inflammation process. Hemorrhage has occurred during treatment with antibiotics ranging from 1.5 to 35 days. On the contrary, we assumed that our patient’s SAH occurred shortly before parent artery occlusion. If the SAH occurred two weeks before the presentation of neurologic deficits, during the occurrence of severe headache, the SAH should have been washed out and not be detected from the initial computed tomography scan. We could not confirm whether there was an IIA during the period of severe headache. However, the IIA rupture caused the SAH and acute thrombosis, which led to the MCA occlusion.
Generally, patients with IIA who present with both hemorrhagic and ischemic components may have a poor prognosis. Of the five patients from previous reports and present study, two presented with a disability and one died (Table 1). Hemorrhage and ischemic conditions are on the opposite end of the treatment spectrum, which complicates treatment options and worsens the outcome. We assumed that our patient, fortunately, had enough collateral circulation, which prevented permanent ischemic complications until spontaneous revascularization salvaged the penumbra area.
Herein, we report a rare case of IIA, with subsequent development of SAH, followed by cerebral infarction. Although the treatment for IIA is controversial, the treatment plan should be cautiously discussed with the patient. We could treat the patient with a surgical bypass with trapping of the parent artery while administering antibiotics. In addition, if the patient presents with signs or symptoms of infection, blood culture and echocardiography could be helpful in confirming underlying infectious diseases, such as infectious endocarditis.
REFERENCES
2. Chun JY, Smith W, Halbach VV, Higashida RT, Wilson CB, Lawton MT. Current multimodality management of infectious intracranial aneurysms. Neurosurgery. 2001; Jun. 48(6):1203–13. discussion 1213-4.

3. Gross BA, Puri AS. Endovascular treatment of infectious intracranial aneurysms. Neurosurg Rev. 2013; Jan. 36(1):11–9. discussion 19.

4. Inoue T, Anzai T, Utsumi Y. Early subarachnoid hemorrhage from a bacterial aneurysm of the middle cerebral artery bifurcation following cerebral infarction caused by a septic embolism: case report. No Shinkei Geka. 2006; Oct. 34(10):1051–5.
5. Kannoth S, Thomas SV. Intracranial microbial aneurysm (infectious aneurysm): current options for diagnosis and management. Neurocrit Care. 2009; 11(1):120–9.

6. Krapf H, Skalej M, Voigt K. Subarachnoid hemorrhage due to septic embolic infarction in infective endocarditis. Cerebrovasc Dis. 1999; May–Jun. 9(3):182–4.

7. Matsubara N, Miyachi S, Izumi T, Yamanouchi T, Asai T, Ota K, et al. Results and current trends of multimodality treatment for infectious intracranial aneurysms. Neurol Med Chir (Tokyo). 2015; 55(2):155–62.

8. Peters PJ, Harrison T, Lennox JL. A dangerous dilemma: management of infectious intracranial aneurysms complicating endocarditis. Lancet Infect Dis. 2006; Nov. 6(11):742–8.

9. Saito A, Kawaguchi T, Hori E, Kanamori M, Nishimura S, Sannohe S, et al. Subarachnoid hemorrhage after an ischemic attack due to a bacterial middle cerebral artery dissecting aneurysm: case report and literature review. Neurol Med Chir (Tokyo). 2014; 54(3):196–200.

10. Singla A, Fargen K, Blackburn S, Neal D, Martin TD, Hess PJ, et al. National treatment practices in the management of infectious intracranial aneurysms and infective endocarditis. J Neurointerv Surg. 2016; Jul. 8(7):741–6.

11. Tashima T, Takaki T, Hikita T, Kuroiwa S, Hamanaka N, Takahashi M. Bacterial intracranial aneurysm associated with infective endocarditis: a case showing enlargement of aneurysm size. No Shinkei Geka. 1995; Nov. 23(11):985–9.
12. Yamaguchi S, Sakata K, Nakayama K, Orito K, Ikeda A, Arakawa M, et al. A surgically treated case with a ruptured bacterial aneurysm of the middle cerebral arterial bifurcation following occlusion. No Shinkei Geka. 2004; May. 32(5):493–9.
Fig. 1
Brain computed tomography scan revealed a scanty subarachnoid hemorrhage in the left Sylvian cistern and focal low density around the external capsule.
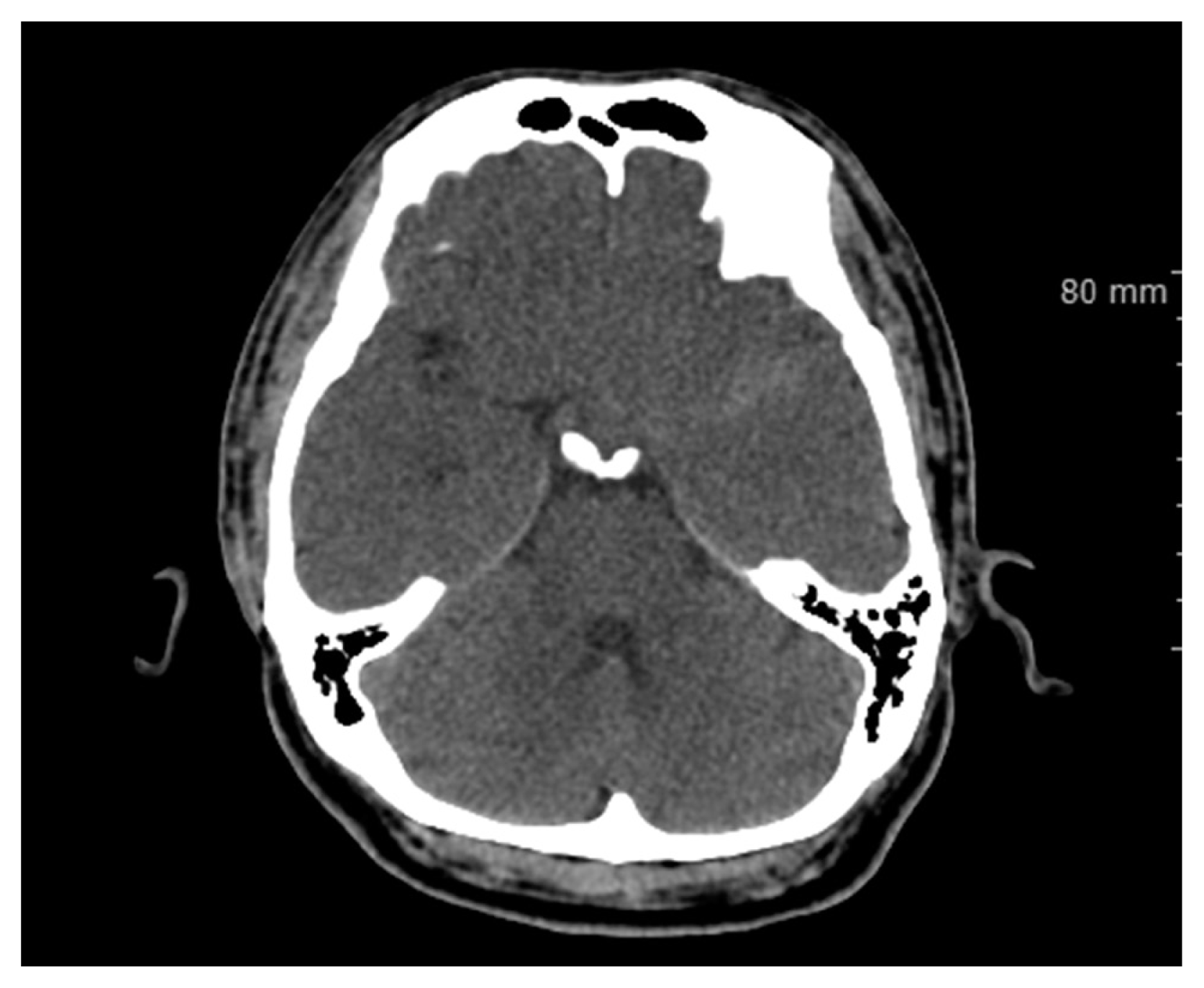
Fig. 2
The left internal carotid artery angiogram showed an obstruction in the middle cerebral artery (arrow in A and B).
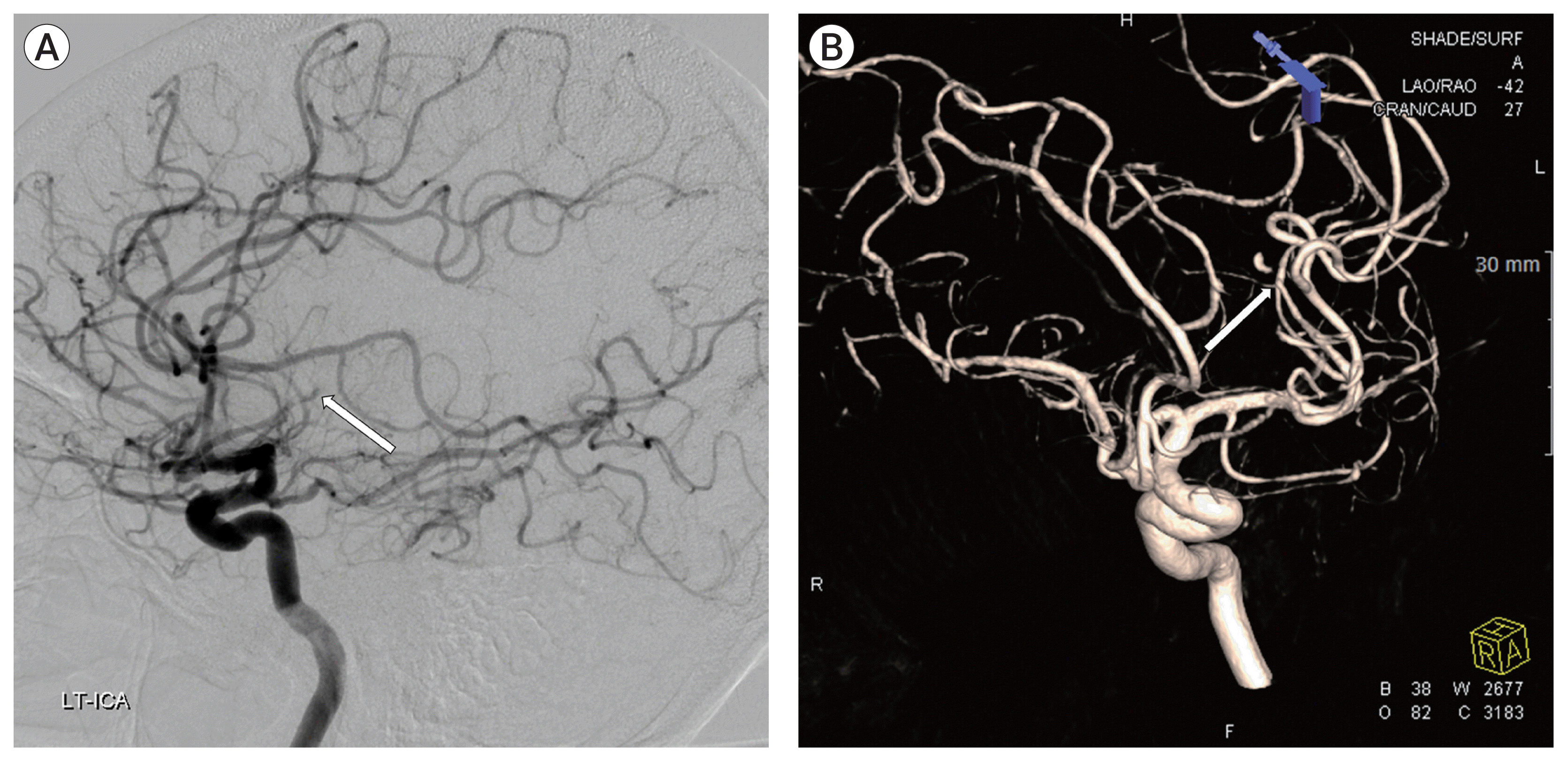
Fig. 3
Diffusion magnetic resonance imaging confirmed acute cerebral infarction around the external capsule and insular lobe, however, the extent of the infarcted lesion remained the same although the neurologic symptoms aggravated (A and B).
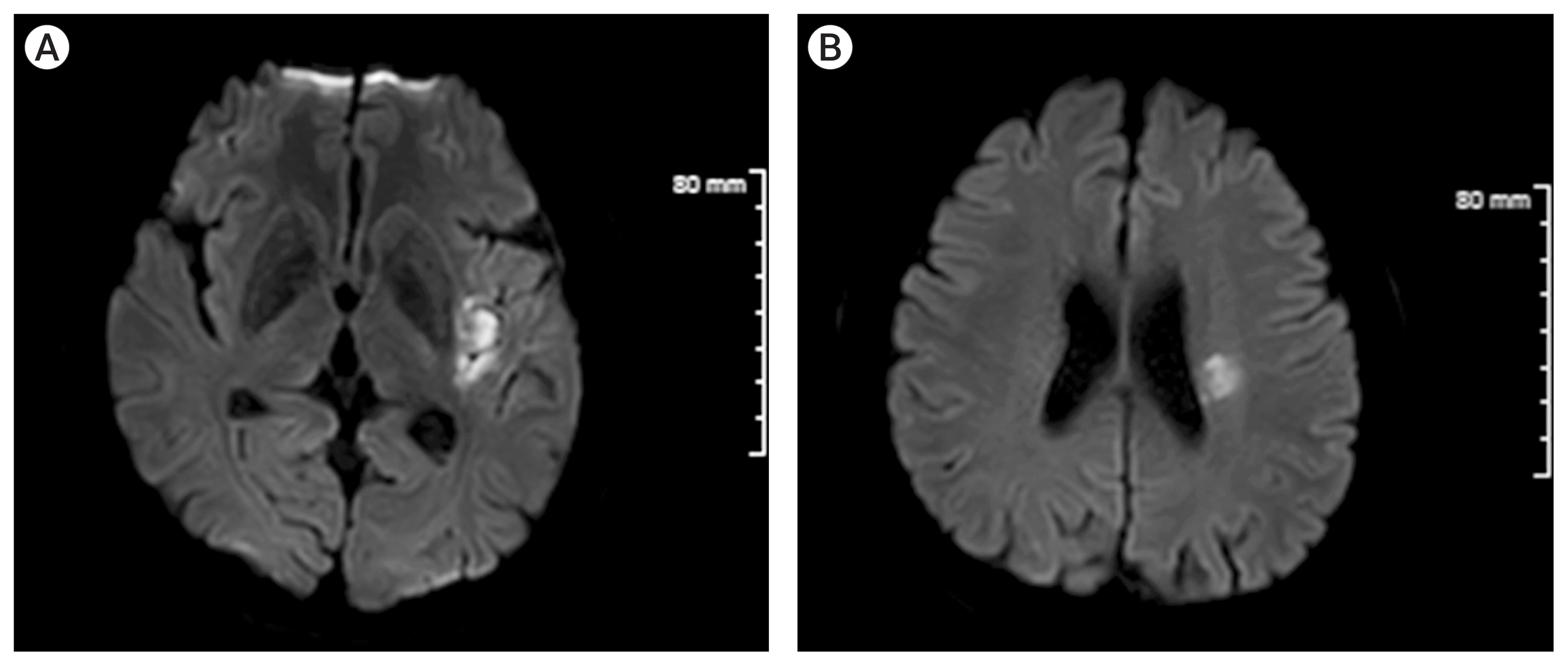
Fig. 4
Moderate mitral regurgitation with vegetation on the mitral valve was confirmed on transthoracic echocardiography, with a diagnosis of infective endocarditis (arrow).
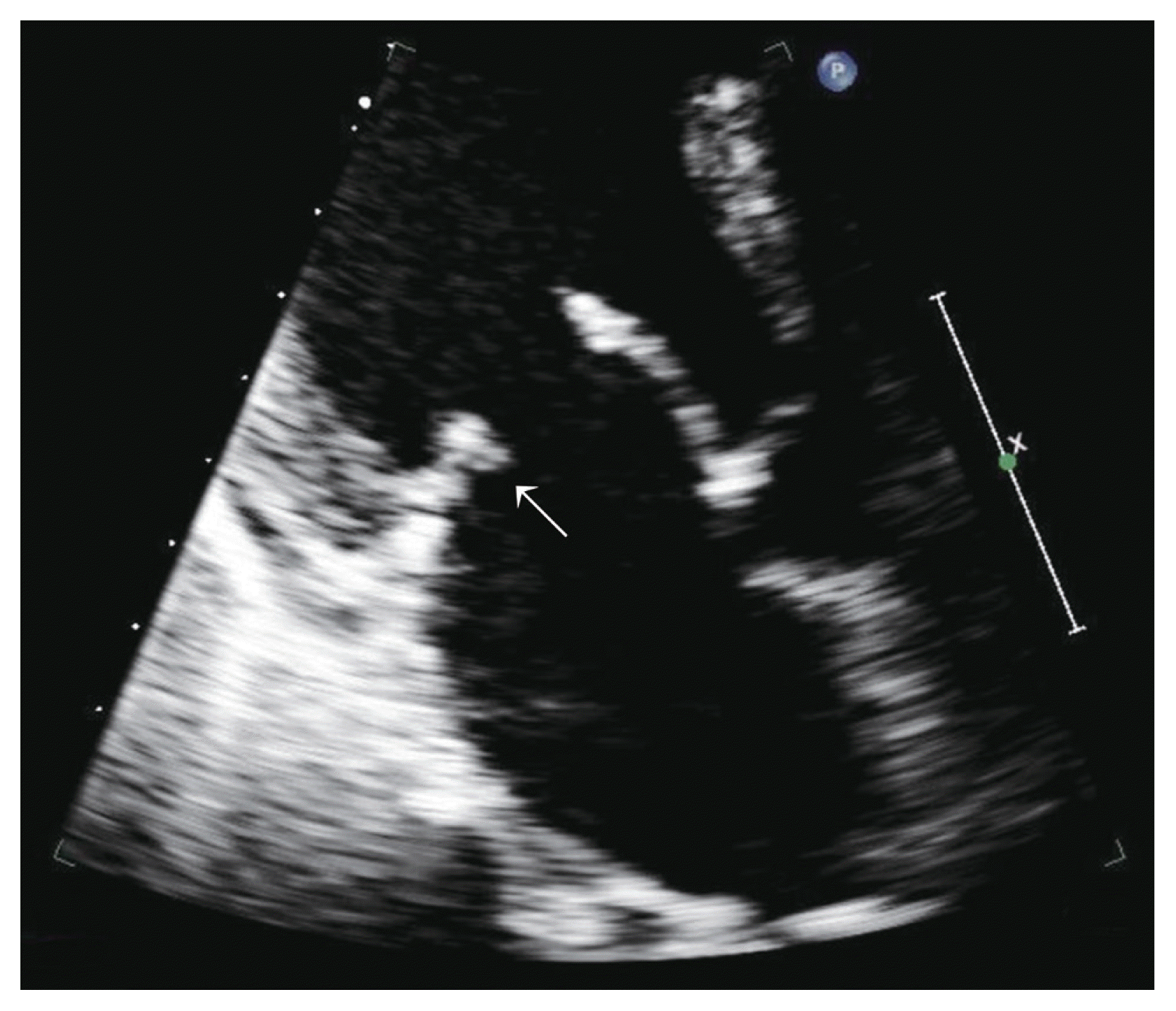
Fig. 5
A brain magnetic resonance angiography, with vessel wall imaging, showed recanalization of the frontal M2 with a fusiform aneurysm on the thirteenth day of admission (A). Follow-up digital subtraction cerebral angiography showed the recanalization of the frontal M2 with a fusiform aneurysm (B). We considered the lesion to be infectious intracranial aneurysm (IIA) caused by infective endocarditis. We performed surgical trapping of the IIA after anastomosis of the frontal branch of the superficial temporal artery and middle cerebral artery.
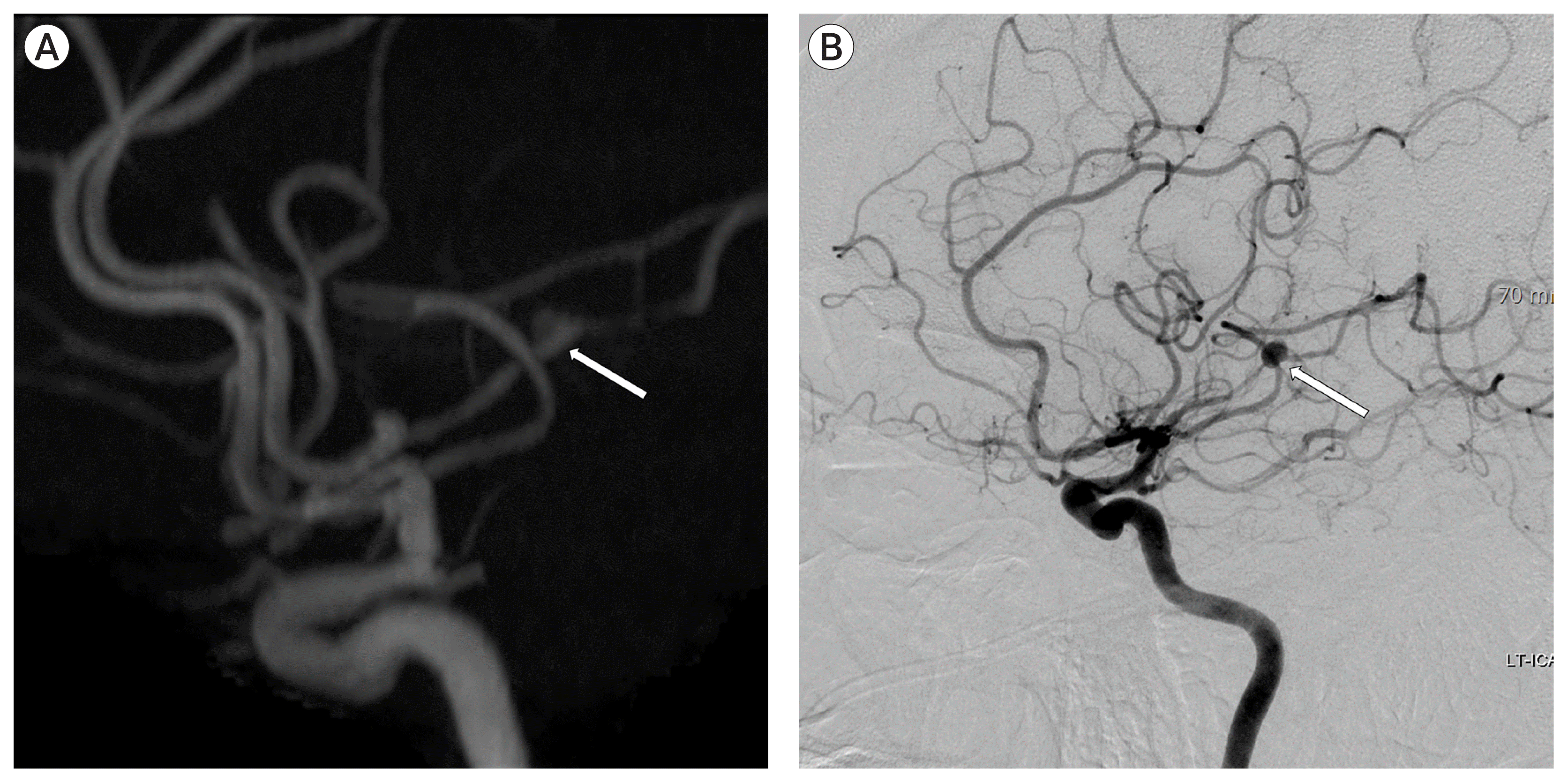
Fig. 6
Yellowish, inflammatory change in the arachnoid membrane was grossly observed around the left Sylvian vein (A). And the aneurysm was enveloped with inflammatory tissues with severe adhesion. Note there are also hemosiderin pigmentation on the arachnoid (B).
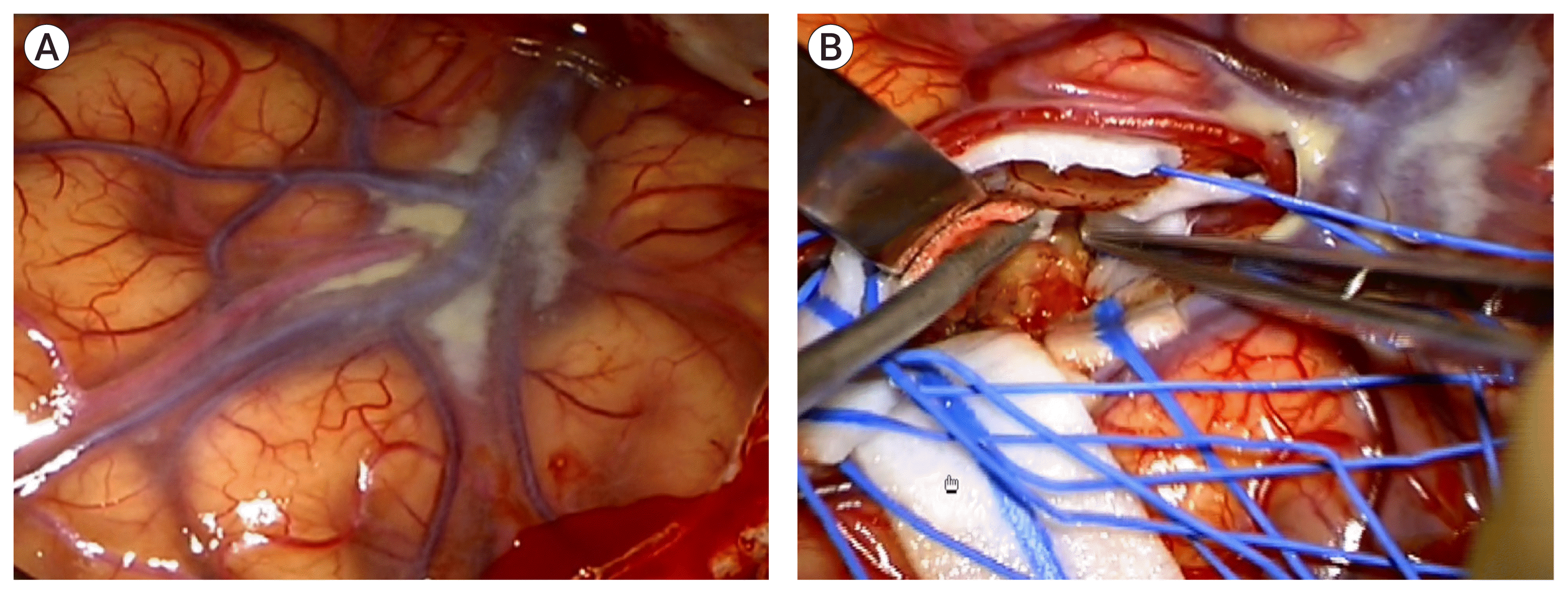
Fig. 7
Trapping of the parent artery using a clip was performed, and postoperative cerebral angiography confirmed the successful anastomosis of the superficial temporal artery-middle cerebral artery bypass (A) without visualization of the aneurysm sac (B).
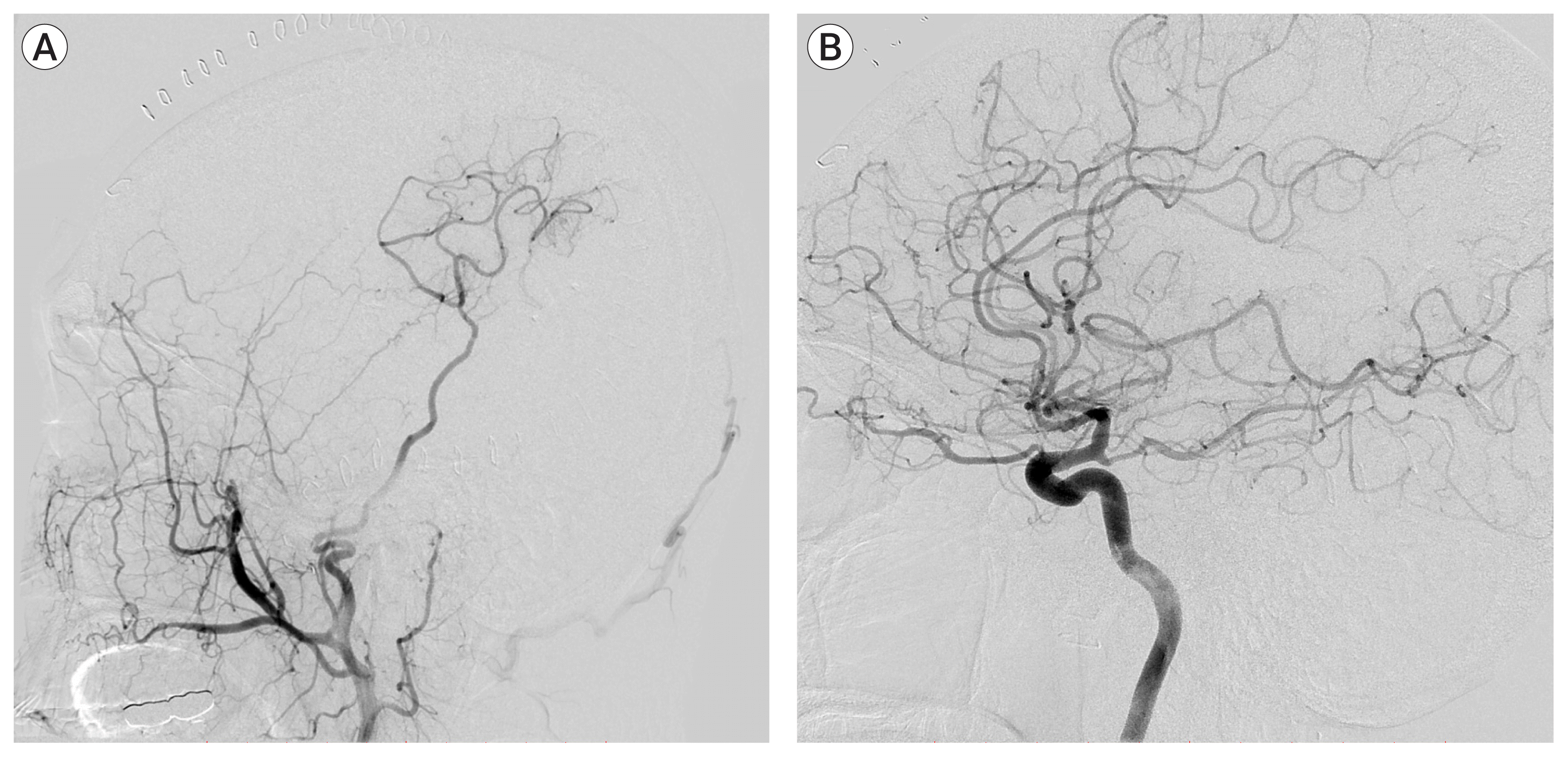
Table 1
Comparison of characteristics of previously published studies and the present study
| Authors & Year | Sex | Age | Initial event | Secondary event | Duration between events | Source of infection | Organism | Findings | Treatment method | Patient outcome |
|---|---|---|---|---|---|---|---|---|---|---|
| Krapf et al. (1999)6) | Male | 56 | Rt. MCA infarction | SAH | 1.5 days | Infective endocarditis | Streptococcus bovis | Necrosis on proximal MCA | Surgical trapping | Hemiparesis |
| Yamaguchi et al. (2004)12) | Female | 56 | Rt. MCA infarction | Subcortical hematoma | 35 days | Infective endocarditis | Streptococcus | Pseudoaneurysm on M2 branch | Surgical trapping with resection | Hemiparesis |
| Inoue et al. (2006)4) | Male | 38 | Lt. MCA infarction | SAH | 4 days | Infective endocarditis | UA | Pseudoaneurysm on M2 branch | Surgical clipping | UA |
| Saito et al. (2014)9) | Female | 78 | Rt. MCA infarction | SAH | 2 days | UA | Gram-positive micrococcus | Dissection of M2 | Autopsy | Expire |
| Present study, 2020 | Male | 41 | SAH | Lt. MCA infarction | Simultaneous | Infective endocarditis | Streptococcus Viridans | Pseudoaneurysm on M2 branch | Surgical bypass with trapping | Fair |




 PDF
PDF Citation
Citation Print
Print



 XML Download
XML Download