Abstract
Background
Peripheral nerve injury rarely occurs in patients with rhabdomyolysis. Based on our experience and previous reports, we consider prolonged immobilization a risk factor for the development of peripheral neuropathy in rhabdomyolysis patients.
Methods
This study analyzed 28 patients with rhabdomyolysis due to prolonged immobilization. We analyzed their demographic and laboratory data, clinical and imaging findings, and outcomes, and compared these factors between patients with and without neuropathy.
Results
Seven of the 28 patients had peripheral neuropathy, including sciatic neuropathy or lumbosacral plexopathy. Compared to those without neuropathy, the patients with neuropathy were younger (p = 0.02), had higher peak creatine kinase (CK) levels (p = 0.02), had higher muscle uptake in bone scans (p = 0.03), and more frequently exhibited abnormal muscle findings in computed tomography (CT) (p = 0.004).
Conclusions
Patients with prolonged immobilization-induced rhabdomyolysis and neuropathy had higher CK levels, increased uptake on bone scans, and more-frequent abnormal muscles on CT than those without neuropathy. These findings indicate that peripheral neuropathy is more likely to develop in patients with severe muscle injury.
Rhabdomyolysis is caused by injury to skeletal muscle, and is characterized by myalgia and swelling of the affected muscles [1]. Peripheral nerve injury is rare in patients with rhabdomyolysis. We recently reviewed the medical records of eight consecutive patients with peripheral neuropathies associated with rhabdomyolysis [2], and found that peripheral neuropathy occurred after prolonged immobilization in all patients except one whose etiology was blunt trauma [2].
Several case reports on neuropathy in rhabdomyolysis [3-6] have described the development of neuropathy after a prolonged period of lying or sitting due to drug- or toxin-induced coma. We therefore hypothesized that prolonged immobilization is associated with the development of peripheral neuropathy in rhabdomyolysis of various origins. We compared the characteristics of rhabdomyolysis patients between those with and without neuropathy after prolonged immobilization.
We retrospectively reviewed the medical records of patients with rhabdomyolysis who were admitted to our hospital between March 2013 and April 2018. Rhabdomyolysis was defined as a creatine kinase (CK) level exceeding 10 times the upper limit of normal. We included patients older than 19 years with rhabdomyolysis with a nontraumatic etiology. Patients were excluded if they had pre-existing renal dysfunction, exhibited symptoms and signs of septic shock, or were transferred elsewhere or died before a neurological examination was performed. This study was approved by our hospital review board (approval no. CR-18-062).
We obtained information on patient demographics (age, sex, weight, and height), the possible etiology of rhabdomyolysis, and inpatient laboratory values including the CK, creatinine, phosphate, calcium, and bicarbonate levels. The etiology of rhabdomyolysis was classified as excessive muscular activity, electrolyte or endocrine abnormality, extreme temperature, infection, drug, or prolonged immobilization. The McMahon score was calculated based on the available laboratory values [7]. On bone scans, the magnitude of muscle uptake was graded visually as follows: grade 0, less than bone radioactivity; 1, equal to bone radioactivity; 2, more than bone radioactivity; and 3, nearly black. A musculoskeletal radiologist blinded to the clinical findings evaluated the muscle changes in the upper thigh and buttocks on abdominopelvic computed tomography (CT) scans. Diffuse muscle swelling and a low muscle signal intensity were regarded as abnormal (Fig. 1). We confirmed neuropathy by reviewing the neurological records and electrodiagnostic findings. We also compared patients with and without neuropathy.
The patients with and without neuropathy were compared using the Mann-Whitney U test for continuous variables and the chi-square test for categorical variables. All analyses were conducted using SPSS software (version 19.0, SPSS, Chicago, IL, USA). All data are presented as mean ± standard-deviation values, and probability values of p < 0.05 were considered to indicate statistical significance.
Eighty-nine patients with nontraumatic rhabdomyolysis met the study inclusion criteria. Prolonged mobilization was associated with rhabdomyolysis in 28 of these patients, of who 7 were diagnosed with peripheral neuropathy. All patients presented with weakness and paresthesia in the lower extremities, and electrodiagnostic tests showed the presence of peripheral nerve injury suggesting sciatic neuropathy or lumbosacral plexopathy. There were no neurological deficits in the other 21 patients with prolonged mobilization.
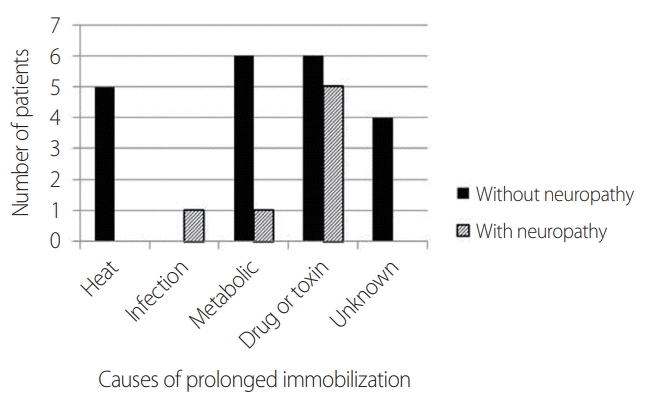
Table 1 lists the demographic features and rhabdomyolysis characteristics of the patients. The patients in the neuropathy group were significantly younger and had significantly higher peak CK levels (26,338 ± 32,014 vs. 59,554 ± 33,006 IU/L, p = 0.02). The magnitude of muscle uptake in the upper extremities on bone scans did not differ between the two groups, whereas that in the lower extremities was significantly higher in the neuropathy group (1.00 ± 0.97 vs. 2.00 ± 0.58, p = 0.03). CT was performed in 6 patients with neuropathy and 11 patients without neuropathy, which revealed abnormal muscles in all of the patients with neuropathy and in 3 of the patients without neuropathy (p = 0.004). The reason for being in a fixed position for a prolonged period did not differ significantly between the two groups (Fig. 2). The other demographic and clinical data also did not differ significantly between the two groups.
During the 5-year period from 2013 to 2018 we encountered eight rhabdomyolysis patients with peripheral neuropathy; this had occurred after prolonged immobilization in seven patients and after blunt trauma in the eighth. Based on our experience and previous reports, we consider prolonged immobilization a risk factor for the development of peripheral neuropathy in rhabdomyolysis patients. In this study we further assessed the characteristics of the patients with neuropathy and found that they were younger, had higher CK levels, and had more-severe muscle injury on CT.
Several mechanisms have been proposed for the peripheral nerve damage that occurs following rhabdomyolysis. First, neuropathy can develop after prolonged lying or sitting due to compression injury, which could explain why neuropathy is common in rhabdomyolysis patients after they have been in a fixed position for a prolonged period. Second, compartment syndrome caused by increased intramuscular pressure can induce compressive neuropathy [5]. Third, several reports have suggested that muscle damage can induce local inflammation and ischemia of adjacent peripheral nerves [6,8].
Renal complications of rhabdomyolysis are common in the elderly [7], but the relationship between age and the risk of rhabdomyolysis remains unclear and depends on the etiology of rhabdomyolysis; for example, statin-induced rhabdomyolysis is common in the elderly [9], while exercise-induced rhabdomyolysis is more common in younger populations [10]. The patients with neuropathy in our study were relatively young, but it remains difficult to explain these results and so further studies are required to confirm the role of age in the development of neuropathy.
Our patients with neuropathy had higher CK levels, higher muscle uptake on bone scans, and abnormal muscles on CT. The magnitude of muscle uptake in the lower extremities was significantly higher in the neuropathy group. These findings may be explained by the peripheral nerve involved, which was the sciatic nerve or lumbosacral plexus in the lower extremities. The blood CK level is generally considered an indirect marker of muscle damage, and the magnitude of muscle uptake on bone scans was correlated with the peak CK level, which may be used to estimate the severity of rhabdomyolysis [11]. CT images showed diffuse areas with low attenuation in the muscle, muscle swelling due to edema, and well-defined intramuscular hypodense foci suggesting muscle necrosis [12]. A muscle with only a mild injury can appear normal on CT, and magnetic resonance imaging has greater sensitivity for detecting abnormalities in muscles [13]. Therefore, the higher CK level, dense uptake on bone scans, and abnormal muscle intensity on CT seen in the present study imply the presence of severe rhabdomyolysis. These findings suggest that the risk of peripheral neuropathy is increased in severe rhabdomyolysis.
This study had several limitations that should be considered when interpreting the findings. First, all of the data were for Korean patients drawn from a single tertiary teaching hospital, and thus might not be generalizable. Second, the study had a retrospective design, with the cause of rhabdomyolysis ascertained from reviews of medical records, which might not have been accurate in all cases. Third, the number of patients was too small to allow the findings to be generalized.
In conclusion, peripheral neuropathy in prolonged immobilization-induced rhabdomyolysis is frequent in patients with severe muscle injury. To our knowledge, this study is the first to elucidate the characteristics of rhabdomyolysis patients with neuropathy.
REFERENCES
2. Seok JI, Lee IH, Ahn KS, Kang GW, Kim JH. Peripheral neuropathies in patients with rhabdomyolysis: clinical characteristics and electrodiagnostic findings in the acute/subacute stage. J Korean Neurol Assoc. 2019; 37:26–29.

3. Maddison P. Acute rhabdomyolysis and brachial plexopathy following alcohol ingestion. Muscle Nerve. 2002; 25:283–285.
4. Nicolle M, Doherty T, Algahtani H. Bilateral femoral neuropathy complicating rhabdomyolysis and acute renal failure. J Clin Neuromuscul Dis. 2005; 6:153–156.

5. Ji JW. Acute compartment syndrome which causes rhabdomyolysis by carbon monoxide poisoning and sciatic nerve injury associated with it: a case report. Hip Pelvis. 2017; 29:204–209.

6. Kim IS, Lee WH, Lim JY. Multiple peripheral neuropathies adjacent to necrotizing myositis related to rhabdomyolysis. AGMR. 2017; 21:31–34.

7. McMahon GM, Zeng X, Waikar SS. A risk prediction score for kidney failure or mortality in rhabdomyolysis. JAMA Intern Med. 2013; 173:1821–1828.

8. Hamel Y, Mamoune A, Mauvais FX, Habarou F, Lallement L, Romero NB, et al. Acute rhabdomyolysis and inflammation. J Inherit Metab Dis. 2015; 38:621–628.

9. Feng Q, Wilke RA, Baye TM. Individualized risk for statin-induced myopathy: current knowledge, emerging challenges and potential solutions. Pharmacogenomics. 2012; 13:579–594.

10. Knapik JJ, O’Connor FG. Exertional rhabdomyolysis: epidemiology, diagnosis, treatment, and prevention. J Spec Oper Med. 2016; 16:65–71.
11. Chang HR, Kao CH, Lian JD, Shu KH, Cheng CH, Wu MJ, et al. Evaluation of the severity of traumatic rhabdomyolysis using technetium-99m pyrophosphate scintigraphy. Am J Nephrol. 2001; 21:208–214.

Fig. 1.
Examples of abnormal computed tomography findings. (A) Axial image showing a low muscle signal intensity and swelling of the right gluteus medius muscle (arrow). (B) Axial image showing diffuse swelling of the right gluteous medius muscle (arrow). GM, gluteus maximus; gm, gluteus medius.
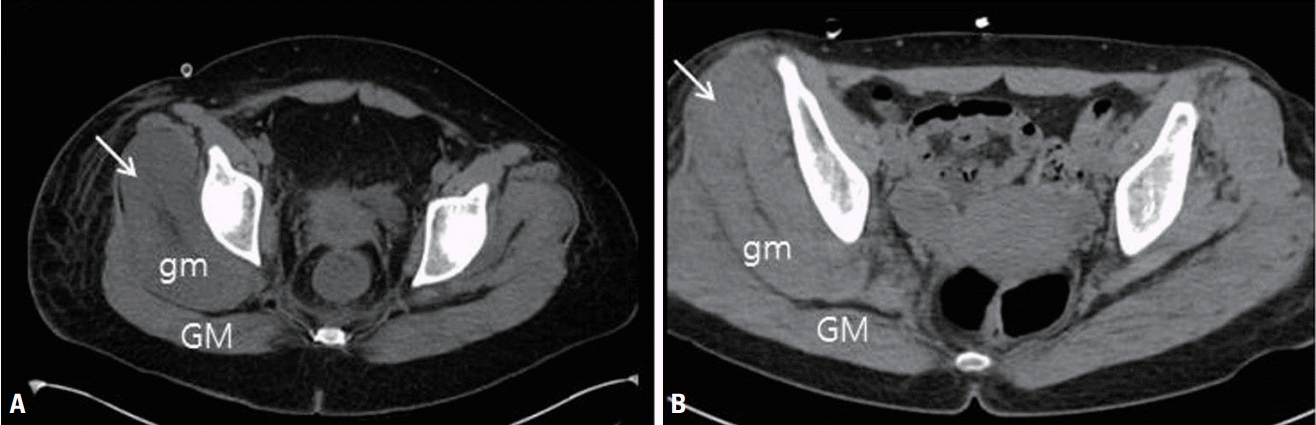
Table 1.
Demographic, clinical, laboratory, and imaging data of the patients
| Characteristic | No neuropathy (n = 21) | Neuropathy (n = 7) | p-valuea |
|---|---|---|---|
| Age (years) | 64.48 ± 19.47 | 40.86 ± 17.17 | 0.02b |
| Sex | |||
| Male | 11 | 4 | 0.83 |
| Female | 10 | 3 | |
| BMI (kg/m2) | 23.46 ± 4.00 | 24.39 ± 4.21 | 0.7 |
| Cause of immobilization | 0.06 | ||
| Heat | 5 | 0 | |
| Infection | 0 | 1 | |
| Metabolic | 6 | 1 | |
| Drug or toxin | 6 | 5 | |
| Unknown | 4 | 0 | |
| Peak CK level (IU/L) | 26,338 ± 32,014 | 59,554 ± 33,006 | 0.02b |
| McMahon score | 9.36 ± 2.64 | 8.29 ± 5.18 | 0.28 |
| Bone scan performed | 16 | 7 | |
| Magnitude of uptake | |||
| Upper extremity | 1.06 ± 0.85 | 1.14 ± 0.69 | 0.86 |
| Lower extremity | 1.00 ± 0.97 | 2.00 ± 0.58 | 0.03b |
| CT (hip and thigh muscles) | 11 | 6 | 0.004b |
| Normal findings | 8 | 0 | |
| Abnormal findings | 3 | 6 | |
| Acute kidney injury | 12 | 3 | 0.67 |
| Renal replacement therapy | 2 | 2 | 0.25 |
| Complete recovery | 0.55 | ||
| CK level | 21 | 7 | |
| Renal function | 18 | 7 |




 Citation
Citation Print
Print



 XML Download
XML Download