Abstract
Background
Methods
Results
ACKNOWLEDGMENTS
REFERENCES
Fig. 1
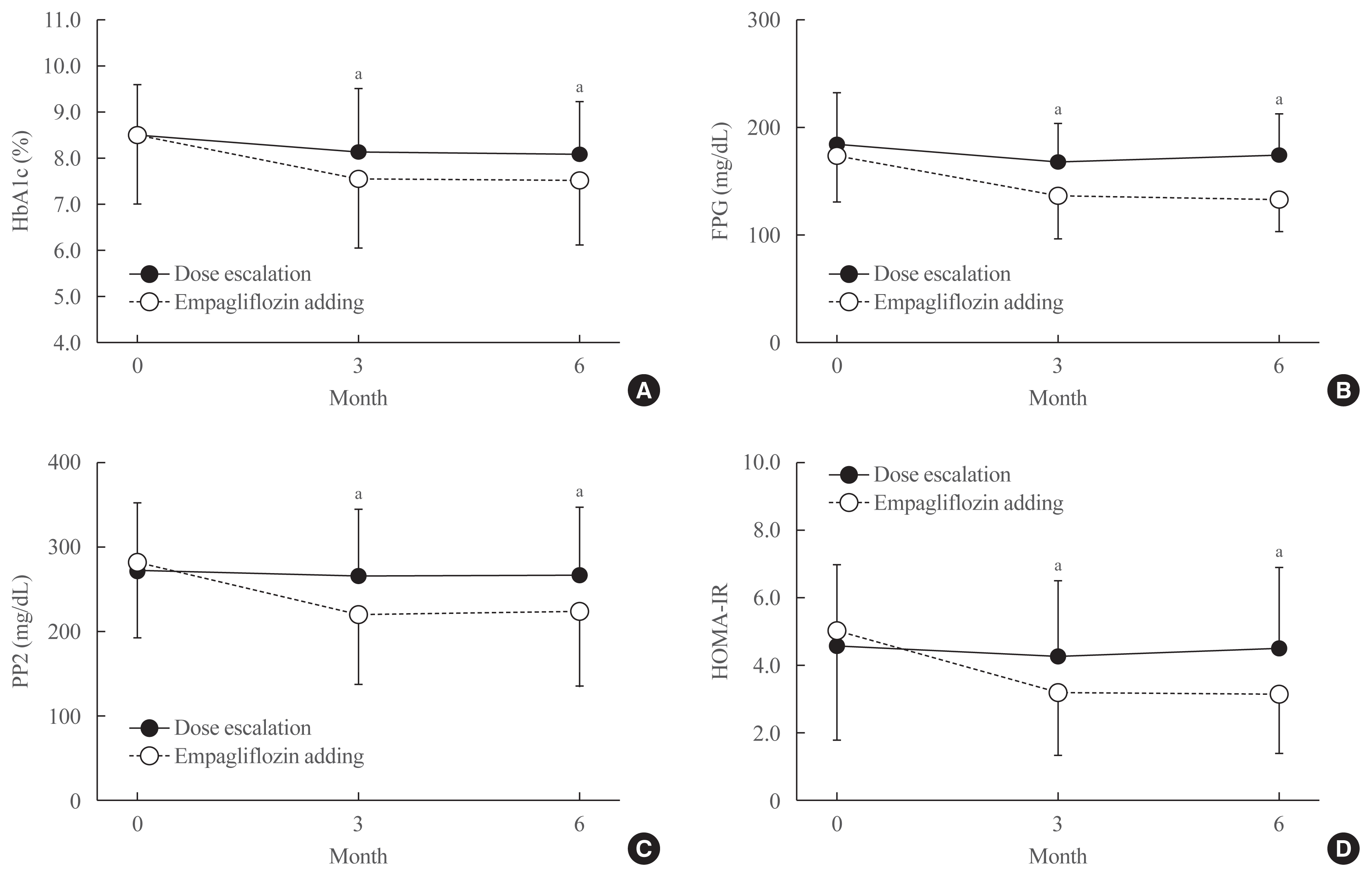
Fig. 2
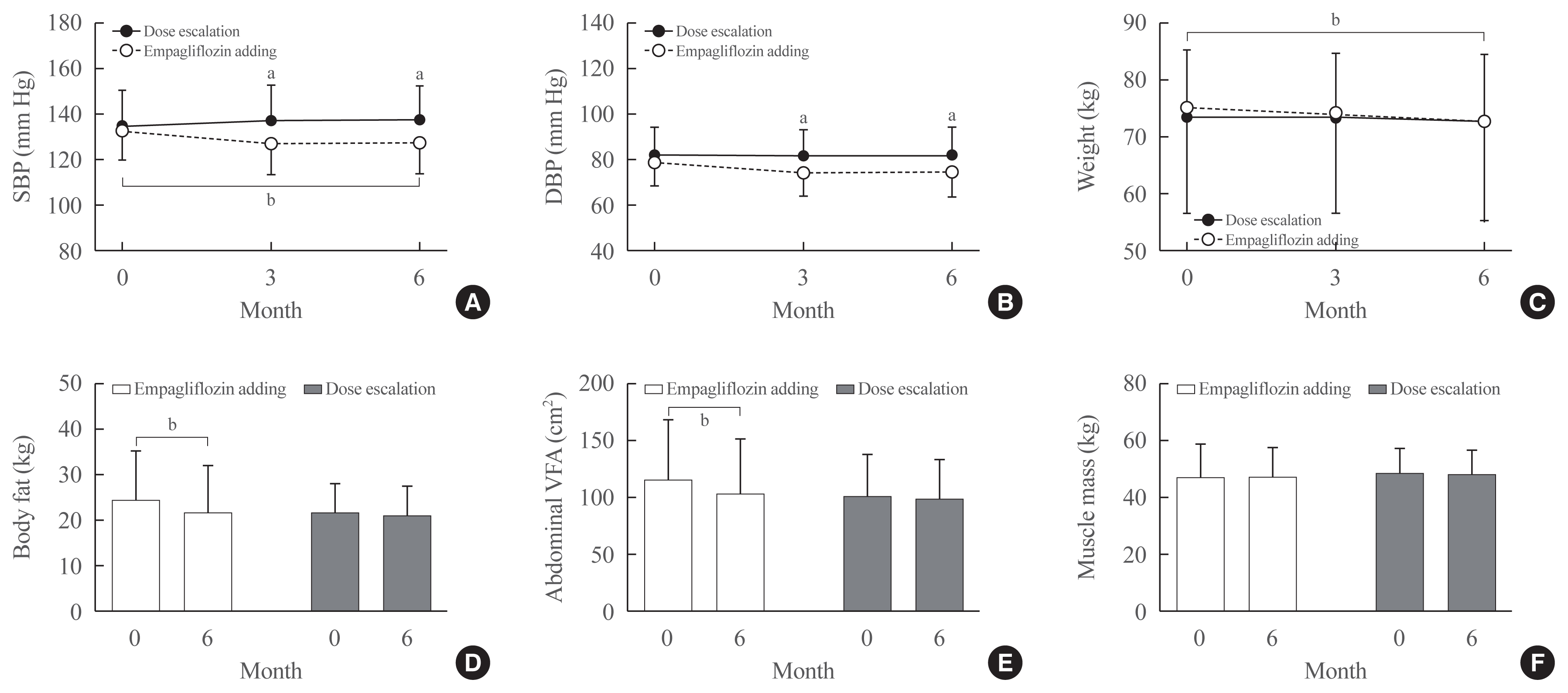
Fig. 3
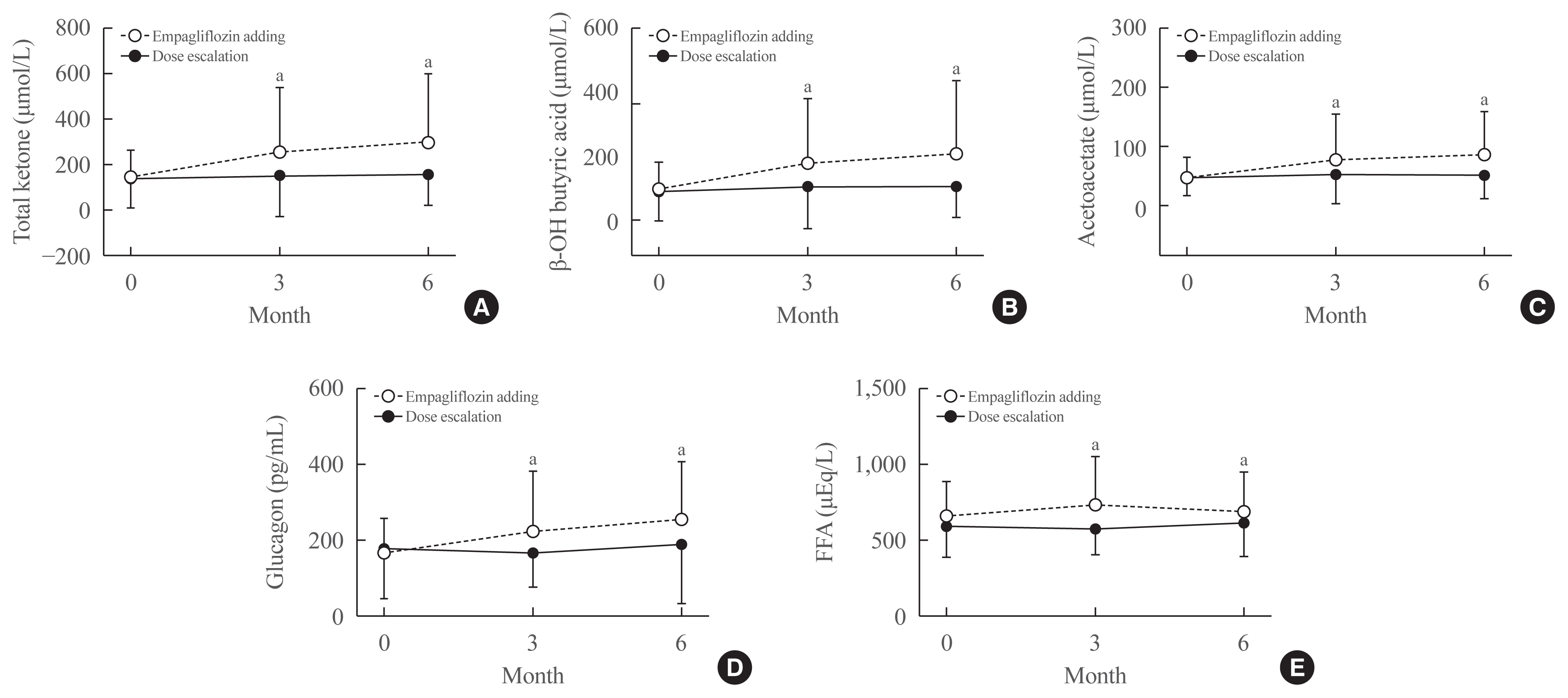
Table 1
NS, not significant; BMI, body mass index; SBP, systolic blood pressure; DBP, diastolic blood pressure; FPG, fasting plasma glucose; PP2, 2-hour postprandial glucose; HbA1c, glycated hemoglobin; HDL-C, high-density lipoprotein cholesterol; LDL-C, low-density lipoprotein cholesterol; AST, aspartate aminotransferase; ALT, alanine aminotransferase; T2D, type 2 diabetes.
Table 2
| Variable | Dose escalation | Empagliflozin add-on | P valuea | ||||
|---|---|---|---|---|---|---|---|
|
|
|
||||||
| Baseline | 6 months | P value | Baseline | 6 months | P value | ||
| SBP, mm Hg | 135.5±15.8 | 138.7±15.2 | 0.286 | 133.6±12.5 | 128.1±13.3 | 0.005b | 0.008b |
|
|
|||||||
| DBP, mm Hg | 82.3±12.6 | 82.0±12.3 | 0.878 | 78.7±9.8 | 74.8±11.0 | 0.009b | 0.120 |
|
|
|||||||
| FPG, mg/dL | 184.9±49.7 | 174.5±39.4 | 0.069 | 174.0±46.7 | 132.9±31.3 | <0.001b | <0.001b |
|
|
|||||||
| PP2, mg/dL | 272.2±82.5 | 267.2±83.0 | 0.616 | 282.9±92.6 | 222.7±88.5 | <0.001b | 0.001b |
|
|
|||||||
| HbA1c, % | 8.5±1.1 | 8.1±1.1 | 0.005 | 8.6±1.6 | 7.6±1.5 | <0.001b | 0.003b |
|
|
|||||||
| Insulin, μIU/mL | 10.0±4.7 | 10.3±4.5 | 0.516 | 12.1±8.2 | 10.0±7.0 | 0.016b | 0.016b |
|
|
|||||||
| C-peptide, ng/mL | 3.0±1.4 | 2.9±1.2 | 0.592 | 3.3±1.6 | 2.7±1.5 | 0.001b | 0.017b |
|
|
|||||||
| Glucagon, pg/mL | 180.0±137.8 | 178.4±131.6 | 0.871 | 167.9±89.8 | 258.0±154.3 | <0.001b | 0.002b |
|
|
|||||||
| Total cholesterol, mg/dL | 168.6±39.1 | 161.7±35.7 | 0.179 | 167.6±41.6 | 157.0±36.5 | 0.030b | 0.730 |
|
|
|||||||
| Triglycerides, mg/dL | 182.5±111.4 | 181.7±159.7 | 0.944 | 169.5±114.9 | 129.0±59.5 | 0.001b | 0.107 |
|
|
|||||||
| HDL-C, mg/dL | 45.8±10.0 | 46.1±11.0 | 0.974 | 49.6±26.2 | 48.6±12.4 | 0.757 | 0.761 |
|
|
|||||||
| LDL-C, mg/dL | 99.5±26.0 | 96.7±26.4 | 0.408 | 95.9±31.3 | 92.3±26.5 | 0.303 | 0.951 |
|
|
|||||||
| AST, IU/L | 32.6±22.3 | 33.8±17.2 | 0.661 | 34.4±17.3 | 28.8±10.9 | 0.002b | 0.023b |
|
|
|||||||
| ALT, IU/L | 35.3±28.7 | 38.2±31.7 | 0.423 | 40.7±29.9 | 33.7±22.1 | 0.015b | 0.023b |
|
|
|||||||
| eGFR, mL/min/1.73 m2 | 96.3±18.7 | 95.6±16.7 | 0.997 | 100.3±18.4 | 100.9±21.0 | 0.703 | 0.772 |
|
|
|||||||
| Creatinine, mg/dL | 0.81±0.18 | 0.81±0.18 | 0.673 | 0.76±0.17 | 0.76±0.19 | 0.623 | 0.520 |
|
|
|||||||
| Sodium, mmol/L | 139.9±2.1 | 139.9±2.2 | 0.671 | 139.7±2.5 | 140.5±1.9 | 0.003b | 0.019b |
|
|
|||||||
| Potassium, mmol/L | 4.5±0.4 | 4.4±0.3 | 0.414 | 4.5±0.4 | 4.5±0.4 | 0.766 | 0.439 |
|
|
|||||||
| Chlorine, mmol/L | 103.2±2.5 | 102.9±2.3 | 0.149 | 102.6±2.5 | 105.2±12.0 | 0.109 | 0.060 |
|
|
|||||||
| FFA, μmol/L | 602.1±207.8 | 619.4±221.6 | 0.336 | 659.4±233.0 | 695.6±261.5 | 0.278 | 0.892 |
|
|
|||||||
| Total ketone, μmole/L | 134.9±121.0 | 158.0±136.8 | 0.487 | 143.0±118.0 | 294.8±296.4 | <0.001b | 0.002b |
|
|
|||||||
| β-Hydroxybutyrate, μmol/L | 90.8±93.3 | 108.0±100.1 | 0.473 | 98.2±84.6 | 207.9±228.3 | <0.001b | 0.003b |
|
|
|||||||
| Acetoacetate, μmol/L | 45.1±29.9 | 50.0±38.5 | 0.688 | 44.8±34.7 | 84.9±71.8 | <0.001b | 0.001b |
|
|
|||||||
| PCr, mg/mmol | 137.3±169.3 | 180.1±295.9 | 0.084 | 165.5±163.8 | 144.4±133.1 | 0.114 | 0.021b |
|
|
|||||||
| MCr, mg/mmol | 44.8±122.4 | 75.4±223.2 | 0.065 | 73.8±138.7 | 50.8±112.7 | 0.039b | 0.006b |
|
|
|||||||
| FE-glucose, % | 2.77±4.47 | 3.55±7.42 | 0.476 | 5.13±9.87 | 30.47±14.95 | <0.001b | <0.001b |
|
|
|||||||
| FE-Na, % | 0.50±0.29 | 0.48±0.34 | 0.047 | 0.46±0.28 | 0.55±0.30 | 0.065 | 0.007b |
|
|
|||||||
| FE-K, % | 9.31±4.61 | 8.87±3.29 | 0.185 | 9.47±4.98 | 9.91±3.66 | 0.569 | 0.188 |
|
|
|||||||
| FE-Cl, % | 0.77±0.40 | 0.79±0.46 | 0.418 | 0.73±0.41 | 0.85±0.48 | 0.085 | 0.066 |
|
|
|||||||
| FE-Ca, % | 0.70±0.52 | 0.60±0.39 | 0.100 | 0.62±0.43 | 0.69±0.38 | 0.182 | 0.053 |
|
|
|||||||
| FE-P, % | 9.64±3.53 | 10.41±4.73 | 0.448 | 9.35±4.31 | 10.08±3.71 | 0.461 | 0.963 |
|
|
|||||||
| Body composition | |||||||
| Body weight, kg | 73.6±12.0 | 73.0±11.8 | 0.141 | 75.5±19.0 | 73.0±18.0 | <0.001b | 0.001b |
| BMI, kg/m2 | 26.4±3.1 | 26.3±2.9 | 0.174 | 27.3±5.2 | 26.3±4.9 | <0.001b | 0.001b |
| Waist circumference, cm | 91.2±8.1 | 91.3±7.4 | 0.474 | 92.6±13.8 | 90.8±12.5 | 0.053 | 0.047b |
| Total body water, L | 38.0±6.8 | 37.8±6.6 | 0.394 | 37.5±8.2 | 37.1±8.1 | 0.515 | 0.925 |
| Lean body mass, kg | 48.8±8.8 | 48.5±8.6 | 0.382 | 47.5±11.5 | 47.6±10.4 | 0.434 | 0.999 |
| Whole body fat mass, kg | 21.9±6.5 | 21.7±6.0 | 0.231 | 24.7±10.9 | 22.3±9.8 | <0.001b | <0.001b |
| Whole body fat percent (%) | 29.7±7.1 | 29.6±6.4 | 0.549 | 31.8±7.9 | 29.8±7.3 | <0.001b | <0.001b |
| Abdominal VFA, cm2 | 102.1±36.1 | 100.5±33.6 | 0.460 | 115.8±52.9 | 104.3±48.8 | <0.001b | <0.001b |
SBP, systolic blood pressure; DBP, diastolic blood pressure; FPG, fasting plasma glucose; PP2, 2-hour postprandial glucose; HbA1c, glycated hemoglobin; HDL-C, high-density lipoprotein cholesterol; LDL-C, low-density lipoprotein cholesterol; AST, aspartate aminotransferase; ALT, alanine aminotransferase; eGFR, estimated glomerular filtration rate; FFA, free fatty acid; PCr, protein/creatinine ratio; MCr, microalbumin/creatinine ratio; FE, fractional excretion; VFA, visceral fat area.




 PDF
PDF Citation
Citation Print
Print



 XML Download
XML Download