This article has been
cited by other articles in ScienceCentral.
Dear Editor,
Hereditary thrombocythemia is a very rare autosomal dominant disorder associated with polyclonal hematopoiesis of the megakaryocytic lineage [
12]. Thrombocythemia 1 (THCYT1), caused by
THPO variant, has been reported in Dutch [
34], Japanese [
56], Polish [
7], Italian [
1], Filipino [
2], and German [
8] families. Here, we report the first case of a Korean boy diagnosed as having THCYT1 using next-generation sequencing (
Table 1). This study was approved by the Institutional Review Board of Keimyung University Dongsan Hospital, Daegu, Korea (approval number: 2019-01-015-002). Informed consent was obtained from all individuals in this study.
In September 2017, a 25-day-old male infant was transferred to Keimyung University Dong San Hospital, following an incidental finding of an abnormal white blood cell (WBC) count. He had congenital defects of his left hand (
Fig. 1A and 1B). One of his two sisters had a history of persistent thrombocythemia of unknown etiology from the age of five months, which was diagnosed at another hospital. His parents and another sister had no past medical history.
Laboratory examination at admission showed a WBC count of 34.6×109/L. His hemoglobin level was 134 g/L, and the platelet count was 197×109/L. The WBC count, determined by manual differential cell count, gradually increased to 57.61×109/L with 3% blasts, 45% segmented cells, 32% lymphocytes, 10% monocytes, 5% eosinophils, and 1% basophils at 39 days of age. Bone marrow analysis at 42 days old showed an increase in the myeloid series with some myeloblasts (5.1%). Megakaryocytes were adequate in number and morphologically normal. We initially suspected juvenile myelomonocytic leukemia; however, clinical and genetic evaluations were inadequate to confirm this diagnosis.
At four months of age, the leukocytosis resolved spontaneously; however, the platelet count increased to >1,000×10
9/L and was sustained at this level (
Fig. 1C). There was no evidence of infection, tissue damage, allergic disease, or autoimmune inflammation associated with secondary thrombocythemia. A second bone marrow analysis revealed slightly increased cellularity with an adequate myeloid:erythroid ratio (2.38:1) and no abnormal finding except for increased megakaryocytes (
Fig. 1D). Genetic evaluation for essential thrombocythemia was performed for
JAK2,
JAK2,
V617F,
MPL, and
CALR, and no pathogenic variant was observed. Treatment with low-dose acetyl salicylic acid (3 mg/kg) was initiated at four months of age.
Targeted exome sequencing was performed to determine the genetic cause of the thrombocythemia in the absence of
JAK2/
MPL/
CALR mutation. Library preparation was performed using the TruSight One sequencing panel (Illumina, San Diego, CA, USA). Massively parallel sequencing was conducted using the NextSeq platform (Illumina, San Diego, CA, USA). Sanger sequencing was performed using the primers F-5′-TCAGGACCCAGACCTGAAAC-3′ and R-5′-CCTACTCTGCCCAGAAGTGC-3′. Sanger sequencing of samples from the patient and his family members confirmed heterozygosity of a splicing pathogenic variant, NM_000460.2: c.13+1G of
THPO for the patient, his father, and the sister with a history of thrombocythemia. His father was presumed to have mosaicism due to a low heterozygous peak. His mother and the other sister possessed the wild-type sequence at c.13+1 of
THPO (
Fig. 1E). Finally, at 11 months of age, the patient was diagnosed as having THCYT1 with
THPO variant (c.13+1G>A). The pedigree suggested that the patient's disease was inherited in an autosomal dominant manner (
Fig. 1F). The patient also showed elevated THPO serum concentration (182 pg/mL, reference value 7–99 pg/mL) in a manual immunoassay (Quest Diagnostics, Valencia, CA, USA). No thrombotic or hemorrhagic events occurred during the 20-month follow-up period.
THPO is an essential cytokine associated with platelet production and plays an important role in the maintenance of early myeloid progenitors [
7]. The multiple upstream AUG codons of the 5′-untranslated region (UTR) in
THPO interrupt translation and prevent overproduction of this cytokine [
7]. Variants at position +1 of the
THPO intron 3 splice donor site result in the skipping of exon 3 and loss of the inhibitory 5′-UTR sequence, thereby disrupting the translational regulation and resulting in increased THPO production and thrombocythemia [
478]. A possible mechanism for the early leukocytosis in patients with thrombocythemia can be related to the additional role of THPO in regulating hematopoietic stem cell viability [
910]. Several studies have reported patients with THCYT1 and coexisting congenital distal limb defects, suggesting that THPO is involved in vasculogenesis, as a regulator of hemangioblast [
18]. Hemorrhagic or thrombotic complications have also been reported occasionally [
37]. Although our patient has been successfully treated with low-dose acetyl salicylic acid, an optimal management strategy for hereditary thrombocythemia is yet to be established.
Figures and Tables
Fig. 1
Clinical features of the thrombocythemia 1 patient. (A) Constricted left hand. (B) Radiographs showing the limb defects of his left hand (missing distal phalanges at digits 2–5) and his normal right hand. (C) Change in the CBC over time. (D) Bone marrow aspirate showing increased megakaryocytes in active form (Wright-Giemsa stain, ×100). (E) Sanger sequencing of THPO in the patients and family members. The red square indicates the position of NM_000460.2: c.13+1G. (F) Pedigree of the family with thrombocythemia 1 and laboratory results. The patient is indicated with an arrow. The filled symbols represent individuals with THPO gene variant. Open symbols represent normal individuals. The individuals are indicated above the corresponding lanes. Age, CBC, serum THPO level (reference value, 7 – 99 pg/mL), and THPO variant are shown.
Abbreviations: CBC, complete blood count; THPO, thrombopoietin; F, forward strand; R, reverse strand; Hb, hemoglobin; WBC, white blood cells; PLT, platelets.
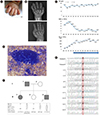
Table 1
Clinical manifestations of reported thrombocythemia 1 families with THPO variant







 PDF
PDF ePub
ePub Citation
Citation Print
Print



 XML Download
XML Download