Abstract
Background
Combination of metformin to reduce the fasting plasma glucose level and an α-glucosidase inhibitor to decrease the postprandial glucose level is expected to generate a complementary effect. We compared the efficacy and safety of a fixed-dose combination of voglibose plus metformin (vogmet) with metformin monotherapy in drug-naïve newly-diagnosed type 2 diabetes mellitus.
Methods
A total of 187 eligible patients aged 20 to 70 years, with a glycosylated hemoglobin (HbA1c) level of 7.0% to 11.0%, were randomized into either vogmet or metformin treatments for 24 weeks. A change in the HbA1c level from baseline was measured at week 24.
Results
The reduction in the levels of HbA1c was −1.62%±0.07% in the vogmet group and −1.31%±0.07% in the metformin group (P=0.003), and significantly more vogmet-treated patients achieved the target HbA1c levels of <6.5% (P=0.002) or <7% (P=0.039). Glycemic variability was also significantly improved with vogmet treatment, estimated by M-values (P=0.004). Gastrointestinal adverse events and hypoglycemia (%) were numerically lower in the vogmet-treated group. Moreover, a significant weight loss was observed with vogmet treatment compared with metformin (−1.63 kg vs. −0.86 kg, P=0.039).
Type 2 diabetes mellitus (T2DM) is characterized by chronic hyperglycemia with insulin resistance and inadequate insulin secretion. It is a major global health problem, with over 382 million people affected in 2013. T2DM is associated with various microvascular and macrovascular complications, which worsen the quality of life and cause more than 5.1 million deaths annually worldwide [1]. Therefore the ultimate goal of managing diabetes mellitus is to reduce the morbidity and the mortality from diabetic complications with intensive glycemic control [2].
Most patients need antidiabetic medication to achieve the recommended target level of glycosylated hemoglobin (HbA1c) in addition to lifestyle modifications. Of the several antihyperglycemic agents that are currently available, metformin—a biguanide—is generally considered the first-choice oral antihyperglycemic drug (OAD) in patients with failure of lifestyle modifications. Metformin is chosen for its antihyperglycemic efficacy, favorable effect on body weight, low risk of hypoglycemia, and cost benefit. However, if metformin monotherapy fails to achieve adequate glycemic control, many guidelines [345] recommend adding a second agent such as sulfonylurea, dipeptidyl peptidase 4 inhibitor, sodium-glucose cotransporter 2 inhibitor or α-glucosidase inhibitor (AGI). Selection of appropriate drug combinations can be tailored depending on the patient's condition, but a combination of drugs with different mechanisms of action is preferred to bring about their synergistic effect [6].
AGIs act by delaying the absorption of carbohydrates in the intestines. They are most effective in reducing postprandial hyperglycemia, and are widely used in many Asian countries where the main staple food is rice. Such a high carbohydrate diet increases the chance of postprandial hyperglycemia, therefore, the use of AGIs could probably attenuate postprandial hyperglycemia [78]. Changes in postprandial glucose (PPG) often precede changes in fasting plasma glucose (FPG) in the natural history of T2DM [910], and while the contribution of FPG to the HbA1c level is dominant in poorly controlled patients with T2DM, the contribution of PPG tends to dominate as HbA1c improves. Thus, managing the levels of PPG as well as FPG is critical in achieving adequate glycemic control [10].
Given this background, a combination of metformin to reduce the FPG level and an AGI to decrease the PPG level is expected to generate a complementary effect. Indeed, miglitol in combination with metformin reduced the HbA1c level more effectively than did metformin monotherapy in patients for whom T2DM was insufficiently controlled by diet alone [11]. Several other studies reported that either acarbose add-on therapy in metformin-treated patients [121314] or metformin add-on therapy in acarbose-treated patients could reduce HbA1c levels effectively [15]. However, there are no reports of studies evaluating the efficacy of a combination of metformin and voglibose as an initial therapy in patients with newly diagnosed T2DM.
One study compared three available AGIs in treatment-naïve patients with T2DM and concluded that voglibose had the best efficacy and safety profile [16], reinforcing the rationale for the development of a fixed-dose combination (FDC) drug of voglibose and metformin. The concept of an oral hypoglycemic FDC—as opposed to loose-pill combinations—could have certain benefits for enhancing patient compliance, which might contribute to improved clinical efficacy, as well as potentially reducing the costs incurred by managing the complications that can arise from suboptimal glycemic control [17]. Therefore, we aimed to investigate vogmet, an FDC of voglibose plus metformin, as an alternative initial therapy for patients with T2DM. Phase 1 and 2 studies were carried out in healthy male volunteers to evaluate the pharmacokinetic and pharmacodynamic properties of metformin and voglibose, and demonstrated that there was no interaction between the two drugs, and that the loose-pill combination and the FDC of voglibose and metformin were biologically equivalent [1819].
In this phase 3 trial, vogmet and metformin were administered orally to subjects with drug-naïve newly diagnosed T2DM for 24 weeks to compare the therapeutic efficacy and safety of vogmet with metformin monotherapy.
This was a multicenter, randomized, double-blind, and parallel-group study conducted in Korean patients in 21 centers across Korea. The study was designed in accordance with the principles stated in the Declaration of Helsinki, and the protocol was reviewed and approved by the independent ethics committees and/or the Institutional Review Board for each of the study sites (IRB no. B-1012/117-001). The study protocol has been registered at ClinicalTrials.gov (https://clinicaltrials.gov; NCT01370707).
Eligible subjects were patients with T2DM and inadequate glycemic control, aged between 20 and 70 years at screening. All the included patients had a body mass index <30 kg/m2, an HbA1c level of 7% to 11%, and all of them were drug-naïve, which means no OAD for more than 7 days for at least 8 weeks prior to the screening visit.
Exclusion criteria for the subjects included: a history of type 1 diabetes mellitus; a requirement for insulin therapy; acute or chronic metabolic acidosis including lactic acidosis or diabetic ketoacidosis; any cardiovascular disease (congestive heart failure, unstable angina, acute myocardial infarction, or arrhythmia); chronic gastrointestinal diseases with digestive and/or absorptive disorders; severe infections, surgical procedures, or severe trauma; a history of substance or alcohol abuse within 1 year; hypersensitivity to biguanides or AGIs; hypopituitarism or hypocortisolism; cancers; hyperthyroidism; requirement for corticosteroid treatment; HbA1c <7% or >11% and FPG >270 mg/dL; serum creatinine >1.5 mg/dL in male subjects and 1.4 mg/dL in female subjects; alanine aminotransferase or aspartate aminotransferase values >2.5-fold the upper limit of normal; systolic blood pressure >150 mm Hg; diastolic blood pressure >90 mm Hg; subjects working night shifts; female subjects with the potential to become pregnant, currently breastfeeding, or not using medically acceptable contraception (e.g., contraceptive pills, hormone injections, intrauterine devices, condoms); or participation in other clinical trials during the 3 months before enrollment.
After they had provided written, informed consent, eligible patients with T2DM at screening were assigned to enter a single-blind placebo run-in period and were reevaluated after 2 weeks. After completing the placebo run-in period, only patients who had an HbA1c level of 7.0% to 11.0%, and adequate compliance (>85% as assessed by tablet counts) during the placebo run-in period were allowed to continue. Patients were stratified based on their HbA1c level (<8% or ≥8%), and were randomized at a 1:1 ratio to receive either vogmet or metformin for 24 weeks.
Drugs were taken orally three times a day (t.i.d.) or two times a day (b.i.d.) before meals, with a single dose escalation at weeks 4, 8, or 12, if the average fasting blood glucose (FBG) level was ≥126 mg/dL, and that dosage was maintained for the remainder of the treatment period. Patients initially receiving vogmet 0.2/250 mg t.i.d. were dose-escalated to vogmet 0.2/500 mg t.i.d., and patients receiving metformin 500 mg b.i.d. were dose-escalated to metformin 500 mg t.i.d., when necessary. To maintain blinding, each patient also took corresponding vogmet-matched or metformin-matched placebo tablets.
Patients were asked to visit their study site every 4 weeks after screening, until the study ended at 24 weeks. They were instructed not to take the study drugs on the morning of their scheduled visit, and fasted for at least 10 hours before it. Patients were also instructed to monitor and record their daily blood level at 2-hour after eating (2h-PPG) using a finger stick glucometer. The average FPG was calculated using the average self-monitored blood glucose (SMBG) level measured before each meal and before bedtime during the 3 days prior to the scheduled hospital visits.
For measuring glycemic variability, we used seven time points of glycemic monitoring, mean SMBG levels, and M-values. The M-value attempts to provide, an expression of both the mean glucose value and the effect of glucose fluctuation in a single numerical value and calculated following previous studies [2021].
The primary endpoint was a change in the HbA1c level at week 24 compared with baseline. The key secondary end points were changes in the levels of HbA1c, FPG, and 2h-PPG at weeks 4, 8, 12, 18, and 24 compared with baseline. Other secondary efficacy end points also included the percentage of patients achieving glycemic targets (HbA1c <7% or <6.5%) at week 24, and glycemic variability at week 8, 18, and 24 compared with baseline.
Safety and tolerability were evaluated by examining several safety parameters. Data from physical examinations, vital signs, laboratory evaluations, and body weight were recorded at each visit. Any adverse events (AEs) were monitored throughout the study along with any adverse drug reactions (ADRs), and serious adverse events (SAEs), and were rated by the investigators for intensity and any association with the study drugs. In particular, the occurrence of gastrointestinal AEs and hypoglycemic events were recorded.
The primary efficacy analysis evaluated whether vogmet was superior to metformin in terms of HbA1c percentage changes from baseline to week 24. The efficacy endpoint was analyzed using analysis of covariance (ANCOVA), with treatments as factors and baseline levels as covariates. The least-squares mean (LS mean) differences and 95% confidence intervals between the two groups were estimated using ANCOVA. Vogmet was judged to be more effective than metformin monotherapy in reducing the level of HbA1c if the P value calculated from ANCOVA for the different treatment groups was <0.05.
Category variables, such as AEs and other safety parameters, were analyzed in terms of the percentage of patients in whom they occurred. Summary statistics were recorded for continuous variables, such as laboratory test results, vital signs and body weight. Differences in mean results before and after drug administration were analyzed using paired Student's t-tests or McNemar's test, and any differences between the two treatment groups were analyzed using unpaired t-tests or Wilcoxon's rank-sum test.
Efficacy analyses were based on the intention-to-treat (ITT) population, which included all patients for whom data were available at baseline and at least one postrandomization time point. Safety and tolerability were analyzed in the all-patients-as-treated population, which included all patients who received at least one dose of a study drug. We used SAS version 9.2 (SAS Institute, Cary, NC, USA) for data analysis, and missing data points were imputed using the last-observation-carried-forward method.
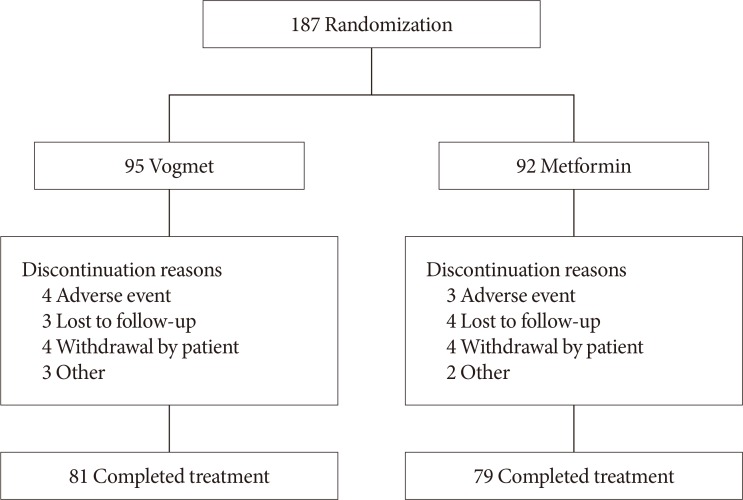
A total of 187 subjects were stratified based on their HbA1c level, and were randomized to a 1:1 ratio (95 vogmet, 92 metformin), to receive one of the study drugs after the 2-week placebo run-in period (Figs. 1 and 2). Selected baseline demographic and clinical data are summarized in Table 1, demonstrating that there were no statistically significant differences between the two treatment groups in their baseline characteristics. After randomization, 160 patients (85.6%) completed the study and 172 patients were included in the ITT population.
We evaluated the changes in HbA1c, FPG, and 2h-PPG levels, and glycemic variability during the 24-week treatment period. At week 24, a decrease in the HbA1c level compared with baseline was statistically significant (P<0.001) for both treatment groups: −1.62%±0.07% in the vogmet group, and −1.31%±0.07% in the metformin group (Fig. 3A). The LS mean treatment difference for the HbA1c level between vogmet and metformin was −0.31% (P=0.003), demonstrating that vogmet, administered orally for 24 weeks, was more effective than metformin monotherapy in lowering the HbA1c level. In the subgroup of subjects whose metformin dosage was 500 mg t.i.d. (vogmet 0.2/500 mg t.i.d. vs. metformin 500 mg t.i.d.), the LS mean treatment difference for the HbA1c level between groups was −0.38% (P=0.001). Likewise, changes in the HbA1c level at each visit (weeks 4, 8, 12, 18, and 24) compared with baseline were statistically significant (P<0.001) for both groups, and significant between-group differences in HbA1c were observed as early as 8 weeks after randomization and continued to increase through week 24 (Fig. 3B).
At week 24, the proportion of subjects reaching the HbA1c target level of <6.5% in the vogmet group was 50%, which was significantly greater (P<0.01) compared with 26.2% in metformin group (Fig. 3C). The proportion of subjects reaching the HbA1c target level of <7% was also significantly larger (P<0.05) in the vogmet group (79.6%) compared with the metformin group (64.3%), indicating that a higher proportion of subjects was able to achieve their target HbA1c levels in the vogmet group compared with the metformin group. During 24-week treatment, the drug compliance was comparable between vogmet group and metformin group (89.0%±17.5% vs. 87.6%±19.5%, P=0.603). The final dosage of metformin was slightly higher in metformin group than vogmet group (1,322.4±166.1 mg vs. 1,208.8±263.1 mg, P<0.001).
Similarly, there was a significant reduction in the FPG level after treatment with the study drugs. Decreases in the FPG levels at each visit compared with baseline were statistically significant (P<0.001) for both groups; again, the changes were statistically greater for the vogmet group compared with the metformin group (P=0.004) at weeks 8, 12, 18, and 24 (Fig. 3D).
Changes in 2h-PPG levels were larger in the vogmet group (−52.89 mg/dL) compared with the metformin group (−45.63 mg/dL); however, the between-group difference was not statistically significant (Fig. 3E).
For the measurements of glycemic variability at the 7-point capillary glucose profile obtained from the SMBG level, we observed less fluctuation in the FBG level after treatment compared with baseline, along with a decrease in FBG concentrations for both treatment groups (Fig. 4A). At week 24, the LS mean differences in the mean FBG concentration calculated from the SMBG were −43.93 mg/dL in the vogmet group and −34.47 mg/dL in metformin group with a significant (P=0.005) between-treatment difference of −9.48 mg/dL (Fig. 4B). The M-values after 24 weeks were 5.73 and 9.85 mg/dL, and the changes in M-value from baseline were −16.1 and −13.03 mg/dL for the vogmet and metformin groups, respectively (Fig. 4C). Again, the decrease in M-value after 24 weeks was significantly (P=0.024) greater in the vogmet group compared with the metformin group (Fig. 4D).
Body weight decreased in both treatment groups. The LS mean differences from baseline in at 24 weeks were −1.63 and −0.86 kg in the vogmet and metformin groups, respectively (Fig. 5). The between-treatment difference was statistically significant (P<0.05).
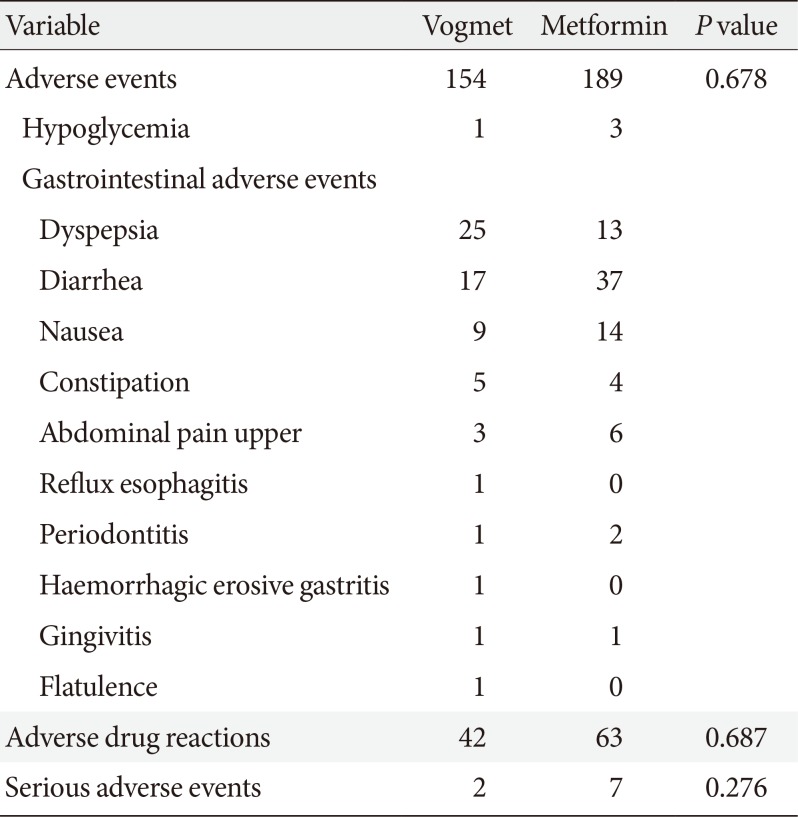
Overall, both vogmet and metformin were well tolerated in this study. The incidence of AEs, ADRs, and SAEs were similar between the two treatment groups (Table 2). The most frequently reported AEs in both groups were gastrointestinal disorders, most of which were mild. Among the gastrointestinal AEs, the most frequent were 25 cases of dyspepsia in the vogmet group and 37 cases of diarrhea in the metformin group. The frequencies of diarrhea, nausea, and upper abdominal pain were numerically lower in the vogmet group compared with the metformin group. No severe hypoglycemic AEs were reported. The incidence of hypoglycemia was slightly higher in the metformin group with three patients versus one receiving vogmet, but the difference was not statistically significant. There were 42 ADRs in the vogmet group and 63 in the metformin group. Two patients in the vogmet group and seven in the metformin group experienced SAEs during the treatment (spinal column stenosis, unstable angina, cellulitis, contusion, open wound, traumatic lung injury, bronchiectasis, mycobacterial infection, or acute appendicitis). However, all were judged to be unrelated to the study drug. Finally, there were no significant abnormalities in laboratory test results, in vital signs, or in the physical examination during the study (data not shown).
This 24-week double-blind study compared the efficacy and safety of vogmet with metformin monotherapy in Korean patients with T2DM and with inadequate glycemic control on diet and exercise. Vogmet provided better reductions in HbA1c and FPG levels from baseline compared with metformin, and greater proportions of patients in the vogmet group met the HbA1c target levels of HbA1c <7% or <6.5% at week 24. These results are similar to the values reported by Goldstein et al. in a study evaluating the effect of initial combination therapy with sitagliptin and metformin [22], illustrating that the combination of metformin and an AGI might be as effective as the combination of metformin with a DPP-4 inhibitor.
Vogmet, a FDC of voglibose and metformin, was developed as an initial therapy for patients with T2DM. Because the combination of an AGI and metformin is ideal in terms of simultaneously controlling two pathological mechanisms of T2DM, the elevation of FPG and postprandial hyperglycemia [823], we believed that vogmet could be a good option in initial therapy for subjects with T2DM. Nevertheless, although the combined administration of two or more active ingredients shows a more effective pharmacological action, there is a problem in that the active ingredients might exacerbate side effects and increase the risk of complications compared with a single drug administration. Consequently, this study was designed to test whether an FDC of voglibose and a lower than conventional dosage of metformin (750 mg/day in vogmet vs. 1,000 to 1,500 mg/day in traditional metformin monotherapy regimen), could manage glycemic parameters effectively, improve patient compliance, and show comparable gastrointestinal AEs profile. The aims of this study were successfully proven.
The greater reduction in glycemic parameters observed in the vogmet group is consistent in lowering the PPG as well as the FPG concentrations contribute to the HbA1c level. Because voglibose is an AGI that can reduce the PPG level very effectively, a greater HbA1c reduction with vogmet treatment can be explained by a synergistic effect of the combination therapy on glycemic control. Further, we could assume that a reduction in the PPG level leads to decreased overall glucose toxicity, which then results in an improved FPG level [1024]. In addition, vogmet treatment also significantly reduced FPG than metformin. In this study, the treatment of vogmet induced more body weight loss than metformin monotherapy did. As body weight reduction can reduce insulin resistance [25], hepatic glucose production might be effectively suppressed and as a result FPG levels were decreased after vogmet treatment. Based on this knowledge, it can be expected to control the progression of diabetes mellitus through simultaneous regulation of the FPG and PPG levels. This would be applicable particularly for patients with early stage T2DM, in light of a report that regulating the PPG concentration has a bigger influence on the HbA1c level in patients even whose blood glucose level is relatively well regulated (HbA1c <7.5%) [24,26].
The measurement of glycemic variability has been considered to contribute macrovascular complications of diabetes mellitus. Glycemic variability is a complex phenomenon that includes both intra- and interday variability, and several approaches have been developed for quantifying it [202728]. In this study, we used seven-time points of glycemic monitoring, mean SMBG levels, and M-values for measuring glycemic variability. The M-value is zero in healthy controls, rising with increasing glycemic variability or poorer glycemic control so that M-values of 0 to 19 are defined as ‘good,’ ≥19 to 32 as ‘fair,’ and ≥32 as ‘poor’ [2021]. All three methods of evaluation showed improved glycemic control for both groups compared with baseline. The M-values changed from 28.14 to 8.16 in the vogmet group, and from 28.57 to 12.43 in metformin group, indicating that they improved from being ‘fair’ to ‘good’ in both treatments, and this suggests that both treatments were effective in reducing glycemic variability. Further, the changes in the mean SMBG levels and M-values were significantly greater in the vogmet group than in the metformin group at week 24, showing that vogmet therapy provided lesser glucose fluctuation.
Similar to other studies using combinations of metformin with an AGI, which reported no increase in gastrointestinal AEs [1415], both treatments in our study were well tolerated overall with no major safety concerns. It is particularly interesting to note that, although some gastrointestinal AEs were expected for both voglibose and metformin, their incidence did not increase with the combination of the two drugs. On the other hand, the incidence decreased with vogmet, possibly because of enhanced tolerability brought about by the lower mean dose of metformin in vogmet FDC. Therefore, it can be expected that vogmet treatment might not only increase drug compliance but also reduce incidence of gastrointestinal AEs. For diarrhea, metformin monotherapy group showed numerically higher incidence than vogmet group. This result would be related with higher dose of metformin in monotherapy group. It is also notable that only one case of hypoglycemia was recorded in the vogmet group, whereas three cases were noted in the metformin group. This data might be related with the potential role of AGI on reducing reactive hypoglycemia [2930]. This supports the idea that vogmet is as safe as metformin therapy.
AGIs are thought generally to have a neutral effect on body weight [3132], whereas metformin is associated with mild weight reduction in overweight and obese patients with T2DM [3334]. Interestingly, in our study there were statistically significant differences in body weight reductions between the vogmet and metformin groups. This was a favorable outcome, adding support for vogmet as a more effective option for considering that majority of individuals with T2DM are overweight or obese. The possible mechanisms of weight reduction in vogmet group might be related to improved microbiota after delayed carbohydrate passage into the small intestine [35], or decreased caloric intake [2436]. However, the underlying mechanism and its clinical relevance need to be examined further.
We also note that over the 24 weeks, the mean metformin dose during study period in the vogmet group was 1,208.81 mg while that of the metformin monotherapy group was 1,322.42 mg (P<0.001). The difference in the metformin dosage was statistically significant, indicating that vogmet showed better glucose lowering efficacy than the monotherapy, with a lower overall dose of metformin. Our data suggest that this lower than usual dosage of metformin in combination with voglibose would be sufficient for effective glycemic control for many patients with newly diagnosed T2DM.
The main limitation in this study was the lack of a voglibose monotherapy group. However, according to previous study performed in Korea to evaluate the efficacy of voglibose monotherapy compared to acarbose monotherapy, the glucose lowering effect of voglibose monotherapy was relatively low (δ change of HbA1c after 8-week treatment was 0.4%) [37]. Therefore, we expected that the decrease in HbA1c level produced by voglibose monotherapy would be much less than in the metformin monotherapy or vogmet groups.
In conclusion, treatment with vogmet as a FDC of voglibose and metformin provided superior glycemic control for HbA1c, 2h-PPG, and FPG levels, greater in weight loss, and less glycemic variability than did metformin monotherapy in this 24-week study on Korean patients with T2DM. Thus, there were clear benefits brought about by the complementary actions of the two active agents. Vogmet was well tolerated with similar incidences of gastrointestinal tract-related AEs and hypoglycemia as with metformin monotherapy, demonstrating that vogmet can be administered safely as an initial therapy to patients with T2DM.
ACKNOWLEDGMENTS
We thank Dong In Koo, Geun Seog Song, Eun Ji Kim, Hyo Ju Park, and Min Ju Hong of clinical research and development, Hyun Wook Park, Chung Hyun Choi, and Jae Hoon of data management and statistical analysis, and Seoung Gyun Han of formulation.
Notes
AUTHOR CONTRIBUTIONS:
Conception or design: S.H.C., S.W.P.
Acquisition, analysis, or interpretation of data: T.J.O., J.M.Y., K.W.M., H.S.S., M.K.L., K.H.Y., Y.D.S., J.Y.P., I.K.J., B.S.C., Y.S.K., S.H.B., I.J.K., D.M.K., S.R.K., K.W.L., J.H.P., I.K.L., T.S.P., S.H.C., S.W.P.
Drafting the work or revising: T.J.O., S.H.C., S.W.P.
Final approval of the manuscript: T.J.O., J.M.Y., K.W.M., H.S.S., M.K.L., K.H.Y., Y.D.S., J.Y.P., I.K.J., B.S.C., Y.S.K., S.H.B., I.J.K., D.M.K., S.R.K., K.W.L., J.H.P., I.K.L., T.S.P., S.H.C., S.W.P.
References
1. International Diabetes Federation. IDF Diabetes Atlas. 6th ed. Brussels: International Diabetes Federation;2013.
2. Inzucchi SE, Bergenstal RM, Buse JB, Diamant M, Ferrannini E, Nauck M, Peters AL, Tsapas A, Wender R, Matthews DR. American Diabetes Association (ADA). European Association for the Study of Diabetes (EASD). Management of hyperglycemia in type 2 diabetes: a patient-centered approach: position statement of the American Diabetes Association (ADA) and the European Association for the Study of Diabetes (EASD). Diabetes Care. 2012; 35:1364–1379. PMID: 22517736.

3. Ko SH, Hur KY, Rhee SY, Kim NH, Moon MK, Park SO, Lee BW, Kim HJ, Choi KM, Kim JH. Committee of Clinical Practice Guideline of Korean Diabetes Association. Antihyperglycemic agent therapy for adult patients with type 2 diabetes mellitus 2017: a position statement of the Korean Diabetes Association. Diabetes Metab J. 2017; 41:337–348. PMID: 29086531.

4. Garber AJ, Abrahamson MJ, Barzilay JI, Blonde L, Bloomgarden ZT, Bush MA, Dagogo-Jack S, DeFronzo RA, Einhorn D, Fonseca VA, Garber JR, Garvey WT, Grunberger G, Handelsman Y, Hirsch IB, Jellinger PS, McGill JB, Mechanick JI, Rosenblit PD, Umpierrez GE. Consensus statement by the American Association of Clinical Endocrinologists and American College of Endocrinology on the comprehensive type 2 diabetes management algorithm: 2018 executive summary. Endocr Pract. 2018; 24:91–120. PMID: 29368965.
5. Diabetes Canada Clinical Practice Guidelines Expert Committee. Lipscombe L, Booth G, Butalia S, Dasgupta K, Eurich DT, Goldenberg R, Khan N, MacCallum L, Shah BR, Simpson S. Pharmacologic glycemic management of type 2 diabetes in adults. Can J Diabetes. 2018; 42(Suppl 1):S88–S103. PMID: 29650116.

6. IDF Clinical Guidelines Task Force. Global guideline for type 2 diabetes. Brussels: International Diabetes Federation;2012.
7. Derosa G, Maffioli P. α-Glucosidase inhibitors and their use in clinical practice. Arch Med Sci. 2012; 8:899–906. PMID: 23185202.
8. Hanefeld M, Schaper F. Acarbose: oral anti-diabetes drug with additional cardiovascular benefits. Expert Rev Cardiovasc Ther. 2008; 6:153–163. PMID: 18248270.

9. Monnier L, Colette C, Dunseath GJ, Owens DR. The loss of postprandial glycemic control precedes stepwise deterioration of fasting with worsening diabetes. Diabetes Care. 2007; 30:263–269. PMID: 17259492.

10. Schrot RJ. Targeting plasma glucose: preprandial versus postprandial. Clin Diabetes. 2004; 22:169–172.

11. Chiasson JL, Naditch L. Miglitol Canadian University Investigator Group. The synergistic effect of miglitol plus metformin combination therapy in the treatment of type 2 diabetes. Diabetes Care. 2001; 24:989–994. PMID: 11375358.

12. Halimi S, Le Berre MA, Grange V. Efficacy and safety of acarbose add-on therapy in the treatment of overweight patients with type 2 diabetes inadequately controlled with metformin: a double-blind, placebo-controlled study. Diabetes Res Clin Pract. 2000; 50:49–56. PMID: 10936668.

13. Phillips P, Karrasch J, Scott R, Wilson D, Moses R. Acarbose improves glycemic control in overweight type 2 diabetic patients insufficiently treated with metformin. Diabetes Care. 2003; 26:269–273. PMID: 12547847.

14. Van Gaal L, Maislos M, Schernthaner G, Rybka J, Segal P. Miglitol combined with metformin improves glycaemic control in type 2 diabetes. Diabetes Obes Metab. 2001; 3:326–331. PMID: 11703422.

15. Wang JS, Huang CN, Hung YJ, Kwok CF, Sun JH, Pei D, Yang CY, Chen CC, Lin CL, Sheu WH. acarbose/metformin fixed-dose combination study investigators. Acarbose plus metformin fixed-dose combination outperforms acarbose monotherapy for type 2 diabetes. Diabetes Res Clin Pract. 2013; 102:16–24. PMID: 23993469.

16. Ismail TSES, Deshmukh SA. Comparative study of effect of alpha glucosidase inhibitors: miglitol, acarbose and voglibose on postprandial hyperglycemia and glycosylated hemoglobin in type-2 diabetes mellitus. Int J Pharma Bio Sci. 2012; 3:337–343.
17. Benford M, Milligan G, Pike J, Anderson P, Piercy J, Fermer S. Fixed-dose combination antidiabetic therapy: real-world factors associated with prescribing choices and relationship with patient satisfaction and compliance. Adv Ther. 2012; 29:26–40. PMID: 22246944.

18. Choi HK, Oh M, Kim EJ, Song GS, Ghim JL, Shon JH, Kim HS, Shin JG. Pharmacokinetic study of metformin to compare voglibose/metformin fixed-dose combination with coadministered voglibose and metformin. Int J Clin Pharmacol Ther. 2015; 53:147–153. PMID: 25546164.

19. Kim HS, Oh M, Kim EJ, Song GS, Ghim JL, Shon JH, Kim DH, Shin JG. Effect of voglibose on the pharmacokinetics of metformin in healthy Korean subjects. Int J Clin Pharmacol Ther. 2014; 52:1005–1011. PMID: 25161160.
20. Monnier L, Colette C, Owens DR. Glycemic variability: the third component of the dysglycemia in diabetes. Is it important? How to measure it? J Diabetes Sci Technol. 2008; 2:1094–1100. PMID: 19885298.

21. Schlichtkrull J, Munck O, Jersild M. The M-valve, an index of blood-sugar control in diabetics. Acta Med Scand. 1965; 177:95–102. PMID: 14251860.
22. Goldstein BJ, Feinglos MN, Lunceford JK, Johnson J, Williams-Herman DE. Sitagliptin 036 Study Group. Effect of initial combination therapy with sitagliptin, a dipeptidyl peptidase-4 inhibitor, and metformin on glycemic control in patients with type 2 diabetes. Diabetes Care. 2007; 30:1979–1987. PMID: 17485570.

23. Rosak C, Mertes G. Critical evaluation of the role of acarbose in the treatment of diabetes: patient considerations. Diabetes Metab Syndr Obes. 2012; 5:357–367. PMID: 23093911.

24. Pan C, Yang W, Barona JP, Wang Y, Niggli M, Mohideen P, Wang Y, Foley JE. Comparison of vildagliptin and acarbose monotherapy in patients with type 2 diabetes: a 24-week, double-blind, randomized trial. Diabet Med. 2008; 25:435–441. PMID: 18341596.

25. Viljanen AP, Iozzo P, Borra R, Kankaanpaa M, Karmi A, Lautamaki R, Jarvisalo M, Parkkola R, Ronnemaa T, Guiducci L, Lehtimaki T, Raitakari OT, Mari A, Nuutila P. Effect of weight loss on liver free fatty acid uptake and hepatic insulin resistance. J Clin Endocrinol Metab. 2009; 94:50–55. PMID: 18957499.

26. Monnier L, Lapinski H, Colette C. Contributions of fasting and postprandial plasma glucose increments to the overall diurnal hyperglycemia of type 2 diabetic patients: variations with increasing levels of HbA(1c). Diabetes Care. 2003; 26:881–885. PMID: 12610053.
27. Monnier L, Colette C. Glycemic variability: should we and can we prevent it. Diabetes Care. 2008; 31(Suppl 2):S150–S154. PMID: 18227477.
28. Nalysnyk L, Hernandez-Medina M, Krishnarajah G. Glycaemic variability and complications in patients with diabetes mellitus: evidence from a systematic review of the literature. Diabetes Obes Metab. 2010; 12:288–298. PMID: 20380649.

29. Koyama H, Ohguchi H, Yagi T, Imaeda K. Nocturnal reactive hypoglycaemia well treated subjectively and objectively with voglibose. BMJ Case Rep. 2017; 2017:220295.

30. Peter S. Acarbose and idiopathic reactive hypoglycemia. Horm Res. 2003; 60:166–167. PMID: 14530603.

31. Holman RR, Cull CA, Turner RC. A randomized double-blind trial of acarbose in type 2 diabetes shows improved glycemic control over 3 years (U.K. Prospective Diabetes Study 44). Diabetes Care. 1999; 22:960–964. PMID: 10372249.

32. Meneghini LF, Orozco-Beltran D, Khunti K, Caputo S, Damci T, Liebl A, Ross SA. Weight beneficial treatments for type 2 diabetes. J Clin Endocrinol Metab. 2011; 96:3337–3353. PMID: 21900381.

33. DeFronzo RA, Goodman AM. Efficacy of metformin in patients with non-insulin-dependent diabetes mellitus. The Multicenter Metformin Study Group. N Engl J Med. 1995; 333:541–549. PMID: 7623902.
34. Ohmura C, Tanaka Y, Mitsuhashi N, Atsum Y, Matsuoka K, Onuma T, Kawamori R. Efficacy of low-dose metformin in Japanese patients with type 2 diabetes mellitus. Curr Ther Res. 1998; 59:889–895.

35. Yang W, Liu J, Shan Z, Tian H, Zhou Z, Ji Q, Weng J, Jia W, Lu J, Liu J, Xu Y, Yang Z, Chen W. Acarbose compared with metformin as initial therapy in patients with newly diagnosed type 2 diabetes: an open-label, non-inferiority randomised trial. Lancet Diabetes Endocrinol. 2014; 2:46–55. PMID: 24622668.

36. Wolever TM, Chiasson JL, Josse RG, Hunt JA, Palmason C, Rodger NW, Ross SA, Ryan EA, Tan MH. Small weight loss on long-term acarbose therapy with no change in dietary pattern or nutrient intake of individuals with non-insulin-dependent diabetes. Int J Obes Relat Metab Disord. 1997; 21:756–763. PMID: 9376887.

37. Jeong IK, Chung JH, Min YK, Lee MS, Lee MK, Kim KW, Chung YE, Park JY, Hong SK, Lee KU. Comparative study about the effects of acarbose and voglibose in type 2 diabetic patients. J Korean Diabetes Assoc. 2002; 26:134–145.
Fig. 2
Overview of study protocol. HbA1c, glycosylated hemoglobin; b.i.d., two times a day; t.i.d., three times a day.
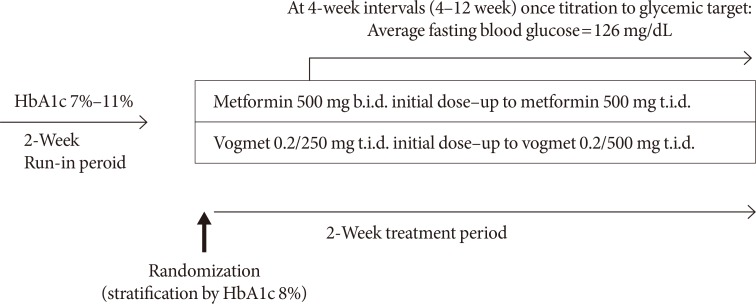
Fig. 3
(A) Least-squares (LS) mean difference in glycosylated hemoglobin (HbA1c) from baseline, and (B) LS mean difference in HbA1c from baseline over time, (C) % of patients with target HbA1c at 24 weeks, (D) LS mean difference in fasting plasma glucose (FPG) from baseline over time, and (E) LS mean difference in 2-hour postprandial glucose (2h-PPG) from baseline at 24 weeks. Data represented by LS mean±standard error. aP<0.05 using analysis of covariance (ANCOVA) adjusted baseline HbA1c and Pearson's chi-square test, bP<0.05 for paired t-test.
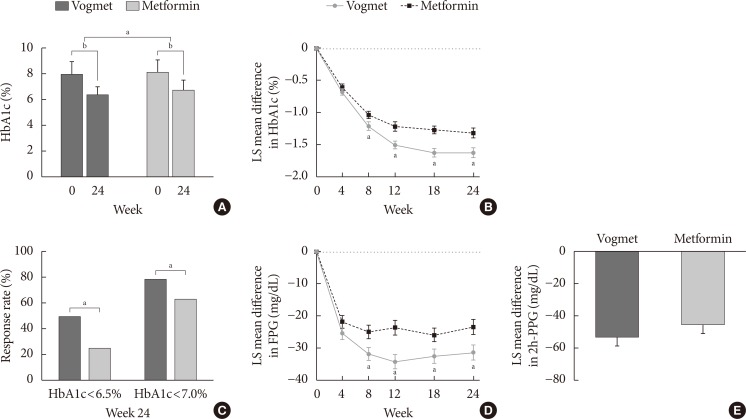
Fig. 4
(A) Changes in self-monitored blood glucose (SMBG), (B) least-squares (LS) mean difference in SMBG at 24 weeks, (C) M-values, and (D) LS mean difference in M-value. Data represented by LS mean±standard error. aP<0.05 using analysis of covariance (ANCOVA).
Fig. 5
Least-squares (LS) mean changes in body weight. Data represented by mean±standard error. aP<0.05 using analysis of covariance (ANCOVA).

Table 1
Baseline characteristics of subjects
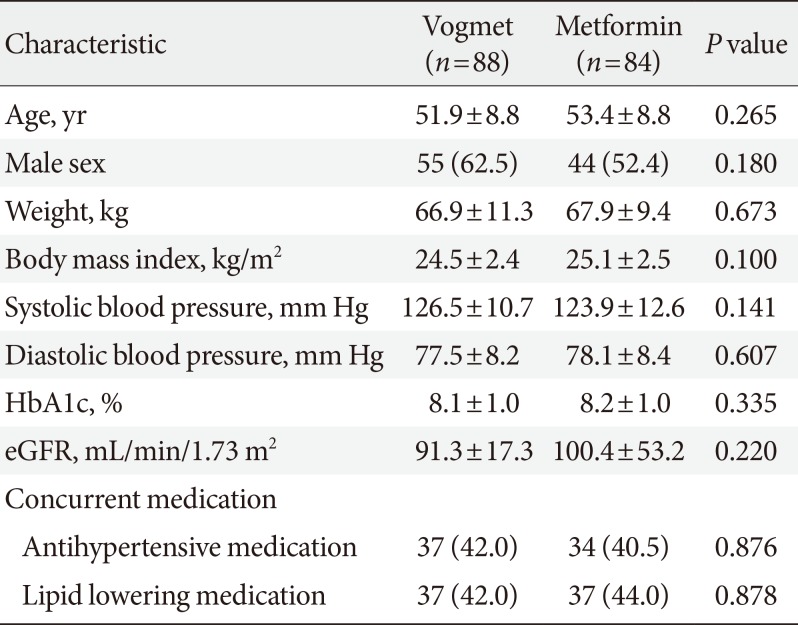
Table 2
Summary of adverse events




 PDF
PDF ePub
ePub Citation
Citation Print
Print



 XML Download
XML Download