This article has been
cited by other articles in ScienceCentral.
To the editor,
Angioedema with eosinophilia (AE) is a rare allergic disease characterized by recurrent episodes of angioedema, fever, weight gain, hypereosinophilia without internal organ damage.
1 AE has been classified into 2 variants, episodic angioedema with eosinophilia (EAE) and non-episodic angioedema with eosinophilia (NEAE).
2 Most cases of AE in Korean and Japanese patients were recognized as non-episodic.
23
Systemic corticosteroid is widely administered to relieve angioedema symptoms while some AE patients show frequent recurrences and steroid dependency, so that novel treatments for AE have been reported including imatinib
4 and mepolizumab.
5 Herein, we report the first case of NEAE treated with reslizumab, a monoclonal antibody against interleukin (IL)-5.
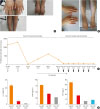
A 41-year-old woman visited the outpatient clinic of pulmonary, allergy, and critical care medicine, Kangdong Sacred Heart Hospital with painful and itchy edema of her extremities. Her peripheral edema was observed in both upper and lower extremities without urticaria and was accompanied by a bodyweight increase of 4 kg for 2 months (
Figure A). She denied any use of drugs and history of insect bite or trauma. The symptoms of allergic rhinitis and conjunctivitis were stationary.
At the first visit, peripheral eosinophil count was 1,140/uL. There was no evidence of parasite infestation in serologic and stool tests. No specific findings were noted in thyroid/liver/kidney function tests, and peripheral blood smear. Serum level of immunoglobulin (Ig) M was 77.1 mg/dL which was within the normal limit. The level of anti-nuclear antibody, C1 esterase inhibitor, and C1 inactivator activity were within the normal limit. The serum concentrations of IL-5 and eosinophil-derived neurotoxin (EDN) were measured to assess the status of eosinophil activation using commercially available ELISA Kits (IL-5, Abcam, Cambridge, MA, USA; EDN, MBL, Nagoya, Japan). Serum levels of eosinophil cationic protein were quantified by ImmunoCAP (Thermo Fisher Scientific/Phadia, Uppsala, Sweden). Written informed consent was obtained from the patient, and the study was approved by the Institutional Review Board of Kangdong Sacred Heart Hospital (KANDONG 2018-03-010-004).
The patient was being treated with intravenous steroids (dexamethasone 15 mg/day) for 4 days, and oral prednisolone was prescribed for 5 days (20 mg/day). Ten days later, she visited the outpatient clinic for aggravated angioedema. Peripheral eosinophil count rose to 1,300/uL. The second high-dose steroid therapy was performed, and oral prednisolone was maintained at a dose of 20 mg/day. The prednisolone dose was tapered from 20 to 15 mg/day for 1 month. However, her symptoms of angioedema relapsed and peripheral eosinophil count rose up to 620/uL.
Due to persistent angioedema, eosinophilia which was refractory to antihistamine and showed failure to taper the steroid, we started reslizumab 170 mg (3 mg/kg body weight) every 4 weeks. Persistent eosinophil activation status and angioedema despite the steroid therapy showed dramatic improvement (
Figure B). Biologics are recommended to be continued if the clinical response is achieved in severe asthma.
6 However, NEAE is known to be transient, we tried to stop reslizumab treatment as symptoms are relieved. After a reslizumab treatment period of 7 months and a follow-up period of 4 months, there were reductions in the daily maintenance dose of prednisolone (15 mg
vs. 0 mg), the peripheral eosinophil count, and the serum levels of IL-5, EDN, and eosinophil cationic protein (
Figure C and D).
Recently, there has been a growing number of studies using eosinophil-targeted biologics (anti-IL-5 antibodies) in eosinophilic disorders including hypereosinophilic syndrome, eosinophilic granulomatosis and polyangiitis, eosinophilic esophagitis, and other disorders.
7 We report here a case of NEAE successfully and safely treated with reslizumab.
In conclusion, this is the first report to treat NEAE with reslizumab. Reslizumab is a treatment option in patients with NEAE.
ACKNOWLEDGMENTS
This work was supported by the National Research Foundation of Korea (NRF) grant funded by the Korea government (MSIP) (No. 2017R1A2B4010060) and a grant No. 2018-1817181800110 from the Kangdong Sacred Heart Hospital Fund.




 PDF
PDF ePub
ePub Citation
Citation Print
Print



 XML Download
XML Download