Abstract
Purpose
The optimal treatment for synchronous liver metastasis (LM) from colorectal cancer (CRC) depends on various factors. The present study was intended to investigate the oncologic outcome according to the time of resection of metastatic lesions.
Methods
Data from patients who underwent treatment with curative intent for primary CRC and synchronous LM between 2004 and 2009 from 9 university hospitals in Korea were collected retrospectively. One hundred forty-three patients underwent simultaneous resection for primary CRC and synchronous LM (simultaneous surgery group), and 65 patients were treated by 2-stage operation (staged surgery group).
Results
The mean follow-up length was 41.2 ± 24.6 months. In the extent of resection for hepatic metastasis, major hepatectomy was more frequently performed in staged surgery group (33.8% vs. 8.4%, P < 0.001). The rate of severe complications of Clavien-Dindo classification grade III or more was not significantly different between the 2 groups. The 3-year overall survival (OS) rate was 85.0% in staged surgery group and 69.4% in simultaneous surgery group (P = 0.013), and the 3-year recurrence-free survival (RFS) rate was 46.4% in staged surgery group and 30.2% in simultaneous surgery group (P = 0.143). In subgroup analysis based on the location of primary CRC, the benefit of staged surgery for OS and RFS was clearly shown in rectal cancer (P = 0.021 and P = 0.015).
Colorectal cancer (CRC) is the third most common malignancy and remains the third leading cause of cancer death in Korea [1]. Liver is the most frequent site of CRC metastasis and its incidence has been reported at approximately 25% at the time of diagnosis and 40%–45% within 2 years after primary resection [234]. Although complete surgical resection is the best option for resectable liver metastases (LM) from CRC, limited patients will have surgically resectable disease. It has been reported that only 10%–25% of patients with synchronous LM from CRC receive radical surgery at the time of initial diagnosis [5].
Optimal treatment for synchronous LM from CRC depends on various factors; a multifactorial treatment strategy, symptoms, location and extent of disease, and the patient's performance status and underlying comorbidities. One-stage surgery, simultaneous resection of primary CRC and metastatic lesion, might be a better treatment option because of the avoidance of a potential delay in surgical therapy for metastatic disease and a reduction in the risk that these metastases could spread if untreated [6]. However, we should consider the increased risk of surgical morbidity. Recent published data have demonstrated the advantages of the perioperative or neoadjuvant chemotherapy, in the aspect of downstaging a patient's disease to a resectable point or to better assess the overall tumor biology [7]. Since target agents such as bevacizumab and cetuximab were introduced, some reports demonstrating the benefit of perioperative chemotherapy over surgery alone, and the potential benefit of adjuvant chemotherapy after the complete resection of metastatic lesion, the timing of surgery for initially resectable metastatic lesion has been hotly debated. However, chemotherapy induced hepatic toxicity cannot be ignored, which increases the risk of perioperative morbidity and mortality, prolongs postoperative recovery, and impairs quality of life [8]. Also, it should be considered that delayed surgery for metastatic disease could lead to an unresectable situation due to disease progression or a missed lesion induced by good tumor response to chemotherapy.
Nowadays, patients with metastatic CRC should be treated by multidisciplinary treatment (MDT) teams comprising surgeons, medical oncologists, radiation oncologists, and radiologists. However, because various factors may affect the decision by MDT teams, a difference in decision can occur even in similar situations of CRC with LM. Thus, the present multicenter retrospective study was conducted in order to minimize the bias from patient selection and intended to investigate long-term oncologic outcomes according to the time of resection of metastatic lesion (simultaneous surgery with the resection of primary lesion vs. staged surgery performed at a certain interval after the resection of primary lesion).
Data from patients who underwent treatment with curative intent for primary CRC and synchronous LM between January 2004 and December 2009 from 9 university hospitals in Korea were collected retrospectively. The pattern of recurrence and follow-up data were obtained using a predetermined data set of individual hospitals, and the follow-up data were censored on December 2013. Among these patients, patients who had extrahepatic metastasis, or, on whom R0 resection was considered impossible to perform (there might be grossly remained tumor in primary or metastatic site after surgery) based on initial abdominal CT scan, were excluded from this study. Also, patients who underwent emergency surgery for their colorectal lesion were excluded from this study. A total of 208 patients on whose initial abdominal CT scan both of colorectal and LM lesion were resectable and who electively underwent surgery for primary CRC and metastatic lesion were enrolled in this study. Of these, 143 patients underwent simultaneous resection for primary CRC and synchronous LM (simultaneous surgery group), and 65 patients were treated by 2-stage operation in which the primary CRC lesion was resected first, and then, metastatic lesions in 2–3 months after 1st operation (staged surgery group) (Fig. 1).
Data collected included patient demographics, primary colorectal surgery, extent of liver resection, pathologic stage and pathologic margin status of metastasectomy (liver resection), pattern and site of recurrence after hepatic surgery, and length of recurrence-free survival (RFS) and overall survival (OS). At this point, the survival time was calculated from the point of colorectal surgery regardless of staged or simultaneous metastasectomy. In order to compare the outcome according to 2 treatment strategies, we divided our patients into 2 groups; one is a group of patients who underwent simultaneous resection for primary CRC and synchronous LM (simultaneous surgery group) and the other is a group of patients who underwent the resection for primary CRC first and then the delayed resection for metastatic lesion after recovery from the resection of primary CRC (staged surgery group).
After obtaining Institutional Review Board (IRB) approval (IRB No. OC18REDI0093) from Incheon St. Mary's Hospital, Kyung Hee University Hospital at Gangdong, Asan Medical Center, Chonnam National University Hwasun Hospital, Korea University Anam Hospital, Dong-A University College of Medicine, and Gil Medical Center to participate in this study, we analyzed the data and clinical information of these 208 patients retrospectively.
Preoperative work-up such as serum CEA at the time of first diagnosis, abdominopelvic CT, chest CT and liver MRI were done based on each hospital's discretion. For hepatic metastatic lesion, liver MRI was used to evaluate the number, extent, and location for metastatic lesions. In patients who underwent neoadjuvant or adjuvant chemotherapy, response to chemotherapy was evaluated by abdomen CT and chest CT every 3 or 4 cycles. After the completion of chemotherapy, patients were examined every 3 months during first 2 years and then every 6 months during the remaining 3- to 5-year schedule with abdomen and pelvic CT and chest CT, or plain chest X-ray. For all patients, follow-up data including information about recurrence and survival were obtained from predetermined data sets from individual hospitals during routine clinical practice.
The primary outcome was in identifying the difference in 3-year OS and 3-year RFS according to each treatment strategy.
The short-term perioperative outcomes including overall postoperative morbidity and recovery course after surgery were analyzed in the overall study population.
All complications occurred during hospital stay and within 30 days after surgery. Postoperative complications were classified as general complication and surgical site (colorectal or hepatic) specific complication. General complication included surgical site infection, postoperative ileus, pulmonary, or cardiac complication. Pelvic abscess or inflammatory change around bowel anastomosis such as fluid collection or abscess formation, chylous ascites, and anastomosis stricture or bleeding were classified into colorectal surgery specific complication, and hepatic abscess or fluid collection and hepatic failure into hepatic surgery specific complication. Postoperative complications were classified using a therapy-oriented 4-level severity grading system; the Clavien-Dindo classification [9]. Postoperative complications with a Dindo classification of III or higher were classified as major complications.
Continuous variables between the 2 groups were compared using paired t-test and expressed as mean ± standard deviation. Continuous variables among the 3 groups were compared using one-way analysis of variance. Categorical variables were analyzed with the chi-square test. Statistical significance was defined as P ≤ 0.05. All statistical analyses were performed using the SPSS ver. 15.0 (SPSS Inc., Chicago, IL, USA).
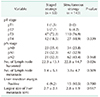
The mean follow-up length was 41.2 ± 24.6 months. The patients in simultaneous surgery group were older than those in staged surgery group (60.0 ± 10.5 vs. 56.4 ± 11.1, P = 0.023). There were no statistical differences in the distribution of gender and American Society of Anesthesiologist (ASA) physical status classification, or the location of primary tumor (colon or rectum). Patients in staged surgery group had more metastatic lesions than those in simultaneous surgery group (2.4 ± 2.0 vs. 1.6 ± 1.1, P = 0.02). Also, neoadjuvant chemotherapy was administered more frequently in staged surgery group than in simultaneous surgery group (53.8% vs. 18.2%, P < 0.001). Most patients who underwent neoadjuvant or adjuvant chemotherapy were treated with FOLFIRI, which is a combination with leucovorin (FOLinic acid), 5-FU (fluorouracil), and IRInotecan or FOLFOX which, itself, is a combination with leucovorin (FOLinic acid), 5-FU (fluorouracil), and oxaliplatin (Table 1).
Operation methods for primary CRC were not significantly different in the 2 groups. However, for the extent of resection for hepatic metastasis, nonanatomical resection was more frequently performed in simultaneous surgery group and major hepatectomy, which was required to resect more than 4 hepatic segments, was more frequently performed in staged surgery group (P < 0.001). Postoperative complications occurred in 12 patients (18.5%) of staged surgery group and 26 (18.2%) of simultaneous surgery group. Of these patients, severe complications of Clavien-Dindo classification grade III or more occurred in 4 patients (6.2%) of staged surgery group and 14 (9.8%) of simultaneous surgery group (P = 0.595). There was no significant difference in surgery site (colon or liver) related complication (P = 0.567) (Table 2).
There were no significant differences in the pathologic results from primary CRC lesion and margin status after hepatic resection in the 2 groups (Table 3).
In univariate analysis, to identify the risk factors for oncologic outcomes, treatment strategy (staged surgery or simultaneous surgery) and N stage were significant factors for OS. And the extent of liver resection, T stage, N stage, and the margin status of hepatic resection were significant factors for RFS (Table 4). In multivariate analysis, there was no significant independent risk factor for OS. Although it was not statistically significant, treatment strategy (staged surgery or simultaneous surgery) showed a tendency to affect OS (P = 0.087; 95% confidence interval [CI], 0.292–1.087). The margin status of hepatic resection was the independent risk factor for RFS (P< 0.001; 95% CI, 0.222–0.634) (Table 5). Fig. 2 shows the OS and RFS according to the treatment strategy. The 3-year OS rate was 85.0% in staged surgery group and 69.4% in simultaneous surgery group (P = 0.013), and the 3-year RFS rate was 46.4 % in staged surgery group and 30.2% in simultaneous surgery group (P = 0.143). In subgroup analysis, dividing into 2 groups based on the location of primary CRC, the benefit of staged surgery for OS and RFS was shown clearly in rectal cancer (P = 0.021 and P = 0.015) (Fig. 3). In multivariate analysis, staged surgery was the independent risk factor for OS and RFS in rectal cancer patients (Table 6).
Table 7 shows the patterns and site of recurrence from our data. After hepatic resection, we found that systemic recurrences including liver or lung occurred in more than half of recurrences.
It has been reported that 10%–25% of synchronous LM from CRC patients are resectable [10]. For resectable synchronous LM from CRC, there are 2 mainstays of therapeutic approach, simultaneous resection or staged resection. Simultaneous resection can prevent the 2nd operation, reduce overall hospital stay, and promptly initiate adjuvant therapy after surgery [11]. On the other hand, there should be concern for increased risk of surgical morbidity. Although staged resection can potentially minimize surgical morbidity, it requires 2 or more operations, results in longer total hospital stay, and could lead to unresectable conditions of LM due to various reasons including disease progression or a missed metastatic lesion due to good response to neoadjuvant chemotherapy [610]. There is no consensus regarding the superiority between these 2 surgical strategies because current results from some investigators have been quite different, and those studies have some biases from referral or institutional differences in treatment policy, or patient selection. To discuss this issue, 2 aspects are necessary to consider; postoperative morbidity and long-term oncological outcomes.
Some authors have demonstrated that staged surgery in patients who would be expected to require major hepatic resection or have excessive surgical stress should be considered. Bolton and Fuhrman [12] reported that surgical death rate was significantly higher if extended lobar resections were necessary, and if concomitant colorectal resection was performed. With this result, they insisted that patients who have complex hepatic metastases at the time of diagnosis of the primary CRC, and who would require extended hepatic lobectomy, should have hepatic resection delayed for at least 3 months after colon resection. One study on surgical morbidity for simultaneous resection for synchronous LM from CRC demonstrated that the frequency of morbidity, and that of anastomotic leakage, seemed to be high after simultaneous resection for synchronous LM from CRC, especially when intraoperative blood loss or operation time increased greatly. Reporting that the predictive factors for postoperative morbidity were intraoperative blood loss and an operation time >8 hours, the authors suggested that staged resection should be considered in cases in which excessive surgical stress including a large amount of blood loss or longer operation time from simultaneous resection would be expected [13]. In the present study, major hepatic resections were more frequently performed in staged surgery group than in simultaneous surgery group (32.8% vs. 8.4%, P < 0.001). This might be because of the difference in number of metastatic lesions between the 2 groups (2.4 ± 2.0 vs. 1.6 ± 1.1, P = 0.02). The rate and site of postoperative morbidity were not significantly different between the 2 groups. Also, the severities of postoperative complications classified with Clavien-Dindo classification were not significantly different between the 2 groups (Table 2). Considering postoperative morbidity, staged surgery might be more appropriate for patients who would be expected to receive major hepatic resection, and simultaneous surgery for those to receive limited nonanatomical resection. A meta-analysis including 24 nonrandomized studies reported that the bilobar distribution, size of LM, and the proportion of major liver resections was found to be higher in the staged surgery group compared to the simultaneous surgery group. Based on the comparable intraoperative parameters, postoperative complications, and survival found between the 2 groups, the authors have suggested that staged surgery group may result in better outcomes, and that simultaneous surgery can only be recommended in patients with limited hepatic disease [14].
One of the important points which must be considered in choosing a therapeutic plan for synchronous LM from CRC is long-term oncologic outcome including RFS and OS. Of course, tumor factors such as the number, the size, the extent of LM, and histopathologic findings of primary CRC may be more important than the therapeutic strategy for stage IV disease [9101516]. In the present study, tumor factors including T and N stage and surgical factors including the extent and margin status of hepatic resection were significant risk factors for RFS (Table 4). Especially, the margin status of hepatic resection was an independent risk factor for RFS (Table 5). However, a well-defined therapeutic plan may be helpful for patients with resectable metastatic disease from CRC. An extensive metaanalysis, which was commented on above, demonstrated that similar oncologic outcomes can be achieved with 2 therapeutic strategies in selected patients [14]. They explained the reason for their conclusion was that most studies only undertook simultaneous resections in patients with limited hepatic disease, and they more frequently performed staged surgery for both bilobar disease and large preoperative size of liver metastasis. Very similar conditions were found in the present study. The authors of the meta-analysis suggested that the point in which staged surgery actually may provide better oncologic outcome than simultaneous surgery in patients with resectable synchronous LM from CRC is debatable [14]. Another study, which was a multicenter international analysis, involving 4 major hepato-biliary centers from the USA, Switzerland, Portugal, and Italy analyzed 1004 patients treated with curative intent surgery for synchronous liver metastasis from CRC [17]. They compared the long-term oncologic outcomes between simultaneous surgery group and staged surgery group. The results showed that the operative strategy for resectable synchronous LM from CRC had no impact on long-term outcomes. They demonstrated that tumor specific factors were more associated with long-term oncologic success. In the present study, there were no statistical differences in local or systemic recurrence rates between simultaneous surgery group and staged surgery group (Table 7). Also, 3-year RFS was not significantly different between the 2 groups (46.4% in staged surgery group vs. 30.2% in simultaneous surgery group, P = 0.143). However, 3-year OS was significantly higher in staged surgery group than in simultaneous surgery group (85.0% vs. 69.4%, P = 0.013) (Fig. 2). Analyzing our data for the subgroup according to the primary tumor locations, there were significantly improved 3-year OS rates and 3-year RFS rates in staged surgery group for rectal cancer with resectable synchronous LM (Table 6, Fig. 3). However, these benefits in oncologic outcomes did not result from colon cancer patients (Fig. 3). The result from present study showed favorable oncologic outcomes from staged surgery for resectable synchronous LM from CRC, especially in rectal cancer patients.
Discussing the therapeutic strategy for stage IV disease from CRC, the role of neoadjuvant or adjuvant chemotherapy should be considered as one of the most important factors. As a development of therapeutic agents for metastatic CRC, long-term oncologic outcomes have been improved [18]. The major goal of systemic chemotherapy before, between, or after resection would increase the likelihood that residual microscopic disease will be eradicated [19]. Neoadjuvant chemotherapy has some potential advantages: earlier treatment of micrometastatic disease, determination of responsiveness to therapy, and avoidance of local therapy for those patients with early disease progression [2021]. However, if the metastatic lesions are completely responsive, neoadjuvant chemotherapy can make it difficult to identify areas for resections. Another potential disadvantage are the inherent risks of disease progression before surgery and the burden of liver toxicity [21]. In the present study, the application rate of neoadjuvant therapy was significantly higher in staged surgery group than in simultaneous surgery group (P < 0.001). That might be because patients in staged surgery group had more metastatic lesions than those in simultaneous surgery group (P = 0.02). Based on our data, in spite of more metastatic lesions in staged surgery group, which is known for poor prognostic factors, the most important point might be that neoadjuvant chemotherapy was more frequently applied to the patients in stage surgery group thus providing the survival benefits in staged surgery group. The data from the EORTC (European Organisation for Research and Treatment of Cancer) study showed quite clearly that nearly all patients were able to tolerate neo-adjuvant chemotherapy. Also, analysis of the progression-free survival curves from the EORTC–EPOC trial shows that the main difference comes after the first 2 months when the curves drop down and then move out in parallel, suggesting that the benefit conferred by perioperative chemotherapy might be a consequence of a reduction in the occurrence of early cancer relapse as a consequence of preoperative chemotherapy [22]. Although it was not clear that this made the difference in long-term oncologic outcome, in cases with large numbers of metastatic lesions, staged surgery should be taken into account as a preferred therapeutic strategy.
This study has some limitations. The major and critical limitation of this study was inherent selection bias. The number of patients who were excluded from staged surgery group due to disease progression after initial colorectal surgery could not be identified. Because cancer progression after initial colorectal resection in staged surgery group might be very important in analyzing the long-term outcomes, careful interpretation of our data might be necessary. At the time of patient enrollment, initial abdominal CT scan was checked to identify the resectability of the metastatic lesion. Thus, any patients with progression after initial colorectal resection in staged surgery group were not lost in present study. Although there was cancer progression, it is reasonable even progression might be considered as a still-resectable condition in the present study. The postoperative complications were not classified in detail and the role of adjuvant chemotherapy in the 2 groups not analyzed. Unfortunately, further information including durations of neoadjuvant treatment and the change of neoadjuvant chemotherapeutic agents before metastasectomy, and so on, except whether or not to perform a neoadjuvant chemotherapy, and kinds of chemotherapeutic agents if the patients received neoadjuvant chemotherapy, were not shown due to the retrospective study design using predetermined data sets from individual hospitals. It was analyzed without consensus about the indication of each therapeutic strategy, selection and reporting bias that was among several institutes, as well as the possible difference of preference to a certain therapeutic strategy. Also, the survival analyses according to tumor-specific factors related to oncologic outcomes were not performed in the present study. However, the result from the present study showed that staged surgery did not appear inferior to simultaneous surgery, but rather, could be superior.
In conclusion, patients with resectable synchronous LM managed with staged surgery had similar perioperative short-term outcomes with simultaneous surgery. On the other hand, oncologic outcomes of staged surgery group had a tendency of longer OS in all CRC with LM and OS and RFS of staged surgery in the subgroup composed of rectal cancer with LM were superior to those with simultaneous surgery group. Although further study including the effect of tumor biology and tumor-specific factors, and the comparison between the 2 strategies in patients with similar disease burdens are needed, staged surgery with or without neoadjuvant chemotherapy should be considered for resectable synchronous LM from CRC as a safe and a fairly promising option.
Figures and Tables
Fig. 2
(A) The 3-year overall survival (OS) rate was 85.0% in the staged surgery group and 69.4% in simultaneous surgery group (P = 0.013); and (B) the 3-year recurrence-free survival rate was 46.4% in the staged surgery group and 30.2% in simultaneous surgery group (P = 0.143). The OS in staged surgery group is significantly better than in simultaneous surgery group.
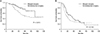
Fig. 3
This figure shows the result of overall survival (OS) and recurrence-free survival (RFS) based on the location of primary CRC. (A) The OS in colon cancer patients was not significantly different between the staged surgery group and simultaneous surgery group (5-year OS rate: 76.6% vs. 55.6%, P = 0.295). However, (B) the OS in rectal cancer patients was significantly better in the staged surgery group than in simultaneous surgery group (5-year OS rate: 73.6% vs. 43.2%, P = 0.021). In the aspect of RFS, the results were almost the same with those for OS. (C) In colon cancer patients, RFS was not significantly different between the 2 groups. However, (D) rectal cancer patients in staged surgery group had better outcome of RFS (5-year RFS rate: 35.3% vs. 20.1%, P = 0.015).

ACKNOWLEDGEMENTS
This study was funded by the Korean Society of Coloproctology. The study sponsor had no involvement in the trial design, collection, analysis, or interpretation of data, or the writing of the report.
References
1. Shin A, Kim KZ, Jung KW, Park S, Won YJ, Kim J, et al. Increasing trend of colorectal cancer incidence in Korea, 1999-2009. Cancer Res Treat. 2012; 44:219–226.

2. Tomlinson JS, Jarnagin WR, DeMatteo RP, Fong Y, Kornprat P, Gonen M, et al. Actual 10-year survival after resection of colorectal liver metastases defines cure. J Clin Oncol. 2007; 25:4575–4580.

3. Van Cutsem E, Cervantes A, Nordlinger B, Arnold D. ESMO Guidelines Working Group. Metastatic colorectal cancer: ESMO Clinical Practice Guidelines for diagnosis, treatment and follow-up. Ann Oncol. 2014; 25 Suppl 3:iii1–iii9.

4. Rees M, Tekkis PP, Welsh FK, O'Rourke T, John TG. Evaluation of long-term survival after hepatic resection for metastatic colorectal cancer: a multifactorial model of 929 patients. Ann Surg. 2008; 247:125–135.
5. Kopetz S, Chang GJ, Overman MJ, Eng C, Sargent DJ, Larson DW, et al. Improved survival in metastatic colorectal cancer is associated with adoption of hepatic resection and improved chemotherapy. J Clin Oncol. 2009; 27:3677–3683.

6. Martin RC 2nd, Augenstein V, Reuter NP, Scoggins CR, McMasters KM. Simultaneous versus staged resection for synchronous colorectal cancer liver metastases. J Am Coll Surg. 2009; 208:842–850.

7. Adam R, Pascal G, Castaing D, Azoulay D, Delvart V, Paule B, et al. Tumor progression while on chemotherapy: a contraindication to liver resection for multiple colorectal metastases? Ann Surg. 2004; 240:1052–1061.
8. Wang Y, Wang ZQ, Wang FH, Yuan YF, Li BK, Ding PR, et al. The role of adjuvant chemotherapy for colorectal l iver metastasectomy after pre-operative chemotherapy: is the treatment worthwhile? J Cancer. 2017; 8:1179–1186.
9. Dindo D, Demartines N, Clavien PA. Classification of surgical complications: a new proposal with evaluation in a cohort of 6336 patients and results of a survey. Ann Surg. 2004; 240:205–213.
10. Martin R, Paty P, Fong Y, Grace A, Cohen A, DeMatteo R, et al. Simultaneous liver and colorectal resections are safe for synchronous colorectal liver metastasis. J Am Coll Surg. 2003; 197:233–241.

11. Yin Z, Liu C, Chen Y, Bai Y, Shang C, Yin R, et al. Timing of hepatectomy in resectable synchronous colorectal liver metastases (SCRLM): Simultaneous or delayed? Hepatology. 2013; 57:2346–2357.

12. Bolton JS, Fuhrman GM. Survival after resection of multiple bilobar hepatic metastases from colorectal carcinoma. Ann Surg. 2000; 231:743–751.

13. Nakajima K, Takahashi S, Saito N, Kotaka M, Konishi M, Gotohda N, et al. Predictive factors for anastomotic leakage after simultaneous resection of synchronous colorectal liver metastasis. J Gastrointest Surg. 2012; 16:821–827.

14. Slesser AA, Simillis C, Goldin R, Brown G, Mudan S, Tekkis PP. A meta-analysis comparing simultaneous versus delayed resections in patients with synchronous colorectal liver metastases. Surg Oncol. 2013; 22:36–47.

15. Minagawa M, Yamamoto J, Miwa S, Sakamoto Y, Kokudo N, Kosuge T, et al. Selection criteria for simultaneous resection in patients with synchronous liver metastasis. Arch Surg. 2006; 141:1006–1012.

16. Mentha G, Majno PE, Andres A, Rubbia-Brandt L, Morel P, Roth AD. Neoadjuvant chemotherapy and resection of advanced synchronous liver metastases before treatment of the colorectal primary. Br J Surg. 2006; 93:872–878.

17. Mayo SC, Pulitano C, Marques H, Lamelas J, Wolfgang CL, de Saussure W, et al. Surgical management of patients with synchronous colorectal liver metastasis: a multicenter international analysis. J Am Coll Surg. 2013; 216:707–716.

18. Adam R, de Gramont A, Figueras J, Kokudo N, Kunstlinger F, Loyer E, et al. Managing synchronous liver metastases from colorectal cancer: a multidisciplinary international consensus. Cancer Treat Rev. 2015; 41:729–741.

19. Yoshidome H, Kimura F, Shimizu H, Ohtsuka M, Kato A, Yoshitomi H, et al. Interval period tumor progression: does delayed hepatectomy detect occult metastases in synchronous colorectal liver metastases. J Gastrointest Surg. 2008; 12:1391–1398.

20. Araujo R, Gonen M, Allen P, Blumgart L, DeMatteo R, Fong Y, et al. Comparison between perioperative and postoperative chemotherapy after potentially curative hepatic resection for metastatic colorectal cancer. Ann Surg Oncol. 2013; 20:4312–4321.

21. Lehmann K, Rickenbacher A, Weber A, Pestalozzi BC, Clavien PA. Chemotherapy before liver resection of colorectal metastases: friend or foe. Ann Surg. 2012; 255:237–247.
22. Nordlinger B, Sorbye H, Glimelius B, Poston GJ, Schlag PM, Rougier P, et al. Perioperative chemotherapy with FOLFOX4 and surgery versus surgery alone for resectable liver metastases from colorectal cancer (EORTC Intergroup trial 40983): a randomised controlled trial. Lancet. 2008; 371:1007–1016.





 PDF
PDF ePub
ePub Citation
Citation Print
Print










 XML Download
XML Download