Dear Editor,
Natural killer (NK)-lymphoblastic leukemia/lymphoma is a rare hematopoietic neoplasm designated by the WHO as an acute leukemia of ambiguous lineage in 2008 and recategorized as a precursor lymphoid neoplasm in 2016 [123]. Patients with this disease are positive for CD56 and immature T-cell markers but negative for B-cell and myeloid markers; moreover, the TCR and IG genes are in the germline configuration [34]. However, the features of this disease overlap with those of other hematological malignancies, and markers specific to NK cell progenitors such as CD94 and CD161 are not commonly tested [456].
Only three patients have met the 2008 WHO criteria [56]. The morphologic and cytogenetic/molecular genetic features of their diseases remain unknown. We report the first Korean patient with NK-lymphoblastic leukemia/lymphoma diagnosed according to the 2016 WHO classification and describe her leukemia-related translocation pattern, genetic profile, and clinical course. Ours is a single case report which does not meet the definition of human subject research, thus was exempted from approval by an Institutional Review Board.
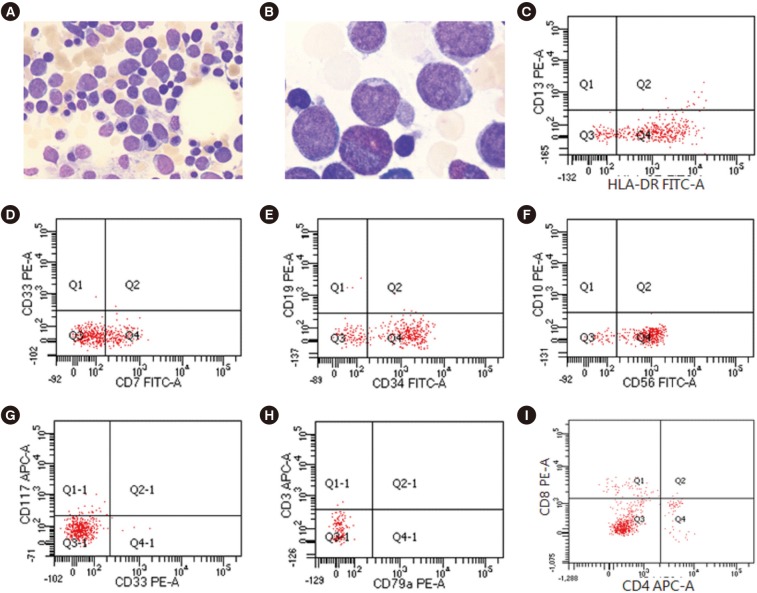
A 51-year-old woman with leukopenia that developed six months prior was referred to Hallym University Sacred Heart Hospital, Anyang, Korea in May 2018. She had experienced transient pancytopenia, presumably caused by a toxic insult to her bone marrow (BM), in February 2017. After spontaneous recovery, her white blood cell count was 1.6×109/L; absolute neutrophil count, 0.25×109/L; hemoglobin, 119 g/L; and platelet count, 176×109/L. Her peripheral blood smear showed a few large lymphocytes with abnormal morphologies. BM aspirate showed a reduced number of normal trilineage hematopoietic cells and an increased number of blasts (63.0% of total nucleated cells) that were large with high nuclear/cytoplasmic ratios, agranular cytoplasm, very fine nuclei with indentation, and conspicuous nucleoli (Fig. 1A and 1B). All cells were negative for peroxidase and Sudan Black B but positive for periodic acid-Schiff; some were also positive for acid alpha-naphthyl acetate esterase. Biopsy revealed BM hypocellularity (10%), while immunohistochemistry showed an increased number of immature cells positive for CD34 and terminal deoxynucleotidyl transferase (TdT). Most blasts were positive for HLA-DR, CD7, CD34, and CD56 but negative for myeloid antigens (CD13, CD33, CD14, CD117, and myeloperoxidase), B-cell antigens (CD10, CD19, CD20, and CD79a), and other T-cell antigens (CD2, cytoplasmic CD3, surface CD3, CD4, CD5, and CD8) (Fig. 1C–1I). The karyotype in BM was 46,XX,−7,del(7)(q35),add(11)(q24),−12,+1mar,+2mar[10]/46,XX[10]. The results of multiplex reverse-transcriptase PCR tests for 28 leukemia-causing chromosomal translocations were all negative. Targeted next-generation sequencing of 54 genes, including BRAF, IKZF1, KRAS, MYD88, NRAS, and TP53, showed no oncogenic mutations; tests for TCRB, IGH, and IGK gene rearrangement showed no dominant clonotypes. Hence, she was diagnosed as having NK-lymphoblastic leukemia/lymphoma. Subsequent physical examinations and computed tomography revealed no organomegaly, lymph node enlargement, or skin lesions. The patient underwent three cycles of fludarabine and cyclophosphamide and remains stable six months post diagnosis.
Jain et al. [7] reported a 23-year-old man with lymphadenopathy with large BM blasts with moderate-to-abundant agranular cytoplasm and inconspicuous or single nuclei. He was positive for CD2, CD5, CD7, CD56, TdT, and HLA-DR and negative for CD34; however, he underwent neither BM karyotyping nor genetic profiling. The authors posited that previously reported leukemias possibly arising from immature NK cells were likely acute myeloid leukemias expressing NK cell markers. Kontogeorgi et al. [8] reported a 24-year-old woman with a brain lesion and infiltrating intermediate-sized agranular lymphocytes expressing TdT and CD56 but lacking myeloid, B-cell, and T-cell markers; she was negative for TCR and IGH gene rearrangement and responded poorly to chemotherapy. Sedick et al. [9] reported a 19-year-old man with lymphadenopathy; intermediate-to-large blasts positive for CD45, HLA-DR, CD16/56, CD2, CD7, CD34, and CD38; variable cytoplasm; nuclear indentation or clefting; and vesicular chromatin. BM karyotyping and FISH showed MYC translocation and TP53 deletion.
Our patient's blast profile and the lack of specific TCR and IG clonotypes ruled out T-acute lymphoblastic leukemia/lymphoma and mixed-phenotype acute leukemia. She was negative for CD2, although NK-lymphoblastic leukemia/lymphoma cases are usually positive for the immature T-cell marker CD2 and pan-T-cell marker CD7 [9]. NK-lymphoblastic leukemia/lymphoma should also be differentially diagnosed from blastic plasmacytoid dendritic cell neoplasm, a rare and deadly myeloid neoplasm that morphologically resembles lymphoblasts with blasts characteristically positive for CD4, CD56, and CD123 [2]. Our patient's blast morphology was not indicative of blastic plasmacytoid dendritic cell neoplasm, and CD4 negativity ruled it out altogether. Nevertheless, she is the first patient to undergo both cytogenetic and molecular genetic profiling.
Acute myeloid/lymphoid leukemias expressing NK cell markers are generally aggressive and have poor prognoses [3]. Three of the four existing cases, including ours, responded well to treatment and had stable clinical courses at the time of reporting. However, long-term follow-up beyond six months remains necessary.
References
1. Swerdlow SH, Campo E, editors. WHO classification of tumours of hematopoietic and lymphoid tissues. Lyon: IARC;2008. p. 155.
2. Swerdlow SH, Campo E, editors. WHO classification of tumours of hematopoietic and lymphoid tissues. 4th ed. Lyon: IARC;2017. p. 213.
3. Oshimi K. Progress in understanding and managing natural killer-cell malignancies. Br J Haematol. 2007; 139:532–544. PMID: 17916099.
4. Koita H, Suzumiya J, Ohshima K, Takeshita M, Kimura N, Kikuchi M, et al. Lymphoblastic lymphoma expressing natural killer cell phenotype with involvement of the mediastinum and nasal cavity. Am J Surg Pathol. 1997; 21:242–248. PMID: 9042293.
5. Petrella T, Bagot M, Willemze R, Beylot-Barry M, Vergier B, Delaunay M, et al. Blastic NK-cell lymphomas (agranular CD4+CD56+hematodermic neoplasms): a review. Am J Clin Pathol. 2005; 123:662–675. PMID: 15981806.
6. Petrella T, Comeau MR, Maynadié M, Couillault G, De Muret A, Maliszewski CR, et al. ‘Agranular CD4+CD56+ hematodermic neoplasm’ (blastic NK-cell lymphoma) originates from a population of CD56+ precursor cells related to plasmacytoid monocytes. Am J Surg Pathol. 2002; 26:852–862. PMID: 12131152.
7. Freud AG, Caligiuri MA. Human natural killer cell development. Immunol Rev. 2006; 214:56–72. PMID: 17100876.
8. Jain S, Kumar R, Purohit A, Pati HP. Precursor NK cell lymphoblastic leukemia/lymphoma-report of a case with literature review. Indian J Hematol Blood Transfus. 2014; 30(S1):283–285. PMID: 25332598.
9. Sedick Q, Alotaibi S, Alshieban S, Naheet KB, Elyamany G. Natural killer cell lymphoblastic leukaemia/lymphoma: Case report and review of the recent literature. Case Rep Oncol. 2017; 10:588–595. PMID: 28868017.
Fig. 1
Immunohistochemical and flow cytometric analyses of the patient's blasts. (A) Large blasts with a high nuclear/cytoplasmic ratio and agranular cytoplasm on the bone marrow aspirate (Wright stain, ×400). (B) Blasts showed very fine nuclei with indentation and conspicuous nucleoli (Wright stain, ×1,000). (C) Flow cytometric analysis showed that the blasts were HLA-DR positive and CD13 negative, (D) CD7 positive and CD33 negative, (E) CD34 positive and CD19 negative, (F) CD56 positive and CD10 negative, (G) CD33 negative and CD117 negative, (H) cytoplasmic CD3 negative and CD79a negative, and (I) CD4 negative and CD8 negative.




 PDF
PDF ePub
ePub Citation
Citation Print
Print



 XML Download
XML Download