1. Hill JD, O’Brien TG, Murray JJ, Dontigny L, Bramson ML, Osborn JJ, Gerbode F. Prolonged extracorporeal oxygenation for acute post-traumatic respiratory failure (shock-lung syndrome). Use of the Bramson membrane lung. N Engl J Med. 1972; 286:629–634.
2. Bartlett RH. Extracorporeal life support: history and new directions. ASAIO J. 2005; 51:487–489.
3. Paden ML, Conrad SA, Rycus PT, Thiagarajan RR, Registry EL. Extracorporeal life support organization registry report 2012. ASAIO J. 2013; 59:202–210.
4. Peek GJ, Mugford M, Tiruvoipati R, Wilson A, Allen E, Thalanany MM, Hibbert CL, Truesdale A, Clemens F, Cooper N, et al. Efficacy and economic assessment of conventional ventilatory support versus extracorporeal membrane oxygenation for severe adult respiratory failure (CESAR): a multicentre randomised controlled trial. Lancet. 2009; 374:1351–1363.
5. Aubron C, Cheng AC, Pilcher D, Leong T, Magrin G, Cooper DJ, Scheinkestel C, Pellegrino V. Infections acquired by adults who receive extracorporeal membrane oxygenation: risk factors and outcome. Infect Control Hosp Epidemiol. 2013; 34:24–30.
6. Haneke F, Schildhauer TA, Schlebes AD, Strauch JT, Swol J. Infections and extracorporeal membrane oxygenation: incidence, therapy, and outcome. ASAIO J. 2016; 62:80–86.
7. Bizzarro MJ, Conrad SA, Kaufman DA, Rycus P; Extracorporeal Life Support Organization Task Force on Infections, Extracorporeal Membrane Oxygenation. Infections acquired during extracorporeal membrane oxygenation in neonates, children, and adults. Pediatr Crit Care Med. 2011; 12:277–281.
8. Schmidt M, Bréchot N, Hariri S, Guiguet M, Luyt CE, Makri R, Leprince P, Trouillet JL, Pavie A, Chastre J, et al. Nosocomial infections in adult cardiogenic shock patients supported by venoarterial extracorporeal membrane oxygenation. Clin Infect Dis. 2012; 55:1633–1641.
9. Undar A, Wang S, Palanzo DA. Impact of polymethylpentene oxygenators on outcomes of all extracorporeal life support patients in the United States. Artif Organs. 2013; 37:1080–1081.
10. Annich G, Lynch W, MacLaren G, Wilson J, Bartlett R. ECMO: Extracorporeal Cardiopulmonary Support in Critical Care. 4th ed. Ann Arbor, MI: Extracorporeal Life Support Organization;2012.
11. Garner JS, Jarvis WR, Emori TG, Horan TC, Hughes JM. CDC definitions for nosocomial infections, 1988. Am J Infect Control. 1988; 16:128–140.
12. Vincent JL, Bihari DJ, Suter PM, Bruining HA, White J, Nicolas-Chanoin MH, Wolff M, Spencer RC, Hemmer M. The prevalence of nosocomial infection in intensive care units in Europe. Results of the European Prevalence of Infection in Intensive Care (EPIC) Study. EPIC International Advisory Committee. JAMA. 1995; 274:639–644.
13. Maillet JM, Guérot E, Novara A, Le Guen J, Lahjibi-Paulet H, Kac G, Diehl JL, Fagon JY. Comparison of intensive-care-unit-acquired infections and their outcomes among patients over and under 80 years of age. J Hosp Infect. 2014; 87:152–158.
14. Schutze GE, Heulitt MJ. Infections during extracorporeal life support. J Pediatr Surg. 1995; 30:809–812.
15. Brown KL, Ridout DA, Shaw M, Dodkins I, Smith LC, O’Callaghan MA, Goldman AP, Macqueen S, Hartley JC. Healthcare-associated infection in pediatric patients on extracorporeal life support: the role of multidisciplinary surveillance. Pediatr Crit Care Med. 2006; 7:546–550.
16. Bihorac A, Efron PA, Ang D, Maier RV, Moldawer LL. Acute kidney injury is associated with nosocomial infections and surgical site infections after trauma. Surg Infect (Larchmt). 2011; 12:S017.
17. Bihorac A, Baslanti TO, Cuenca AG, Hobson CE, Ang D, Efron PA, Maier RV, Moore FA, Moldawer LL. Acute kidney injury is associated with early cytokine changes after trauma. J Trauma Acute Care Surg. 2013; 74:1005–1013.
18. Hoste EA, De Corte W. Clinical consequences of acute kidney injury. Contrib Nephrol. 2011; 174:56–64.
19. Grigoryev DN, Liu M, Hassoun HT, Cheadle C, Barnes KC, Rabb H. The local and systemic inflammatory transcriptome after acute kidney injury. J Am Soc Nephrol. 2008; 19:547–558.
20. Lee DW, Faubel S, Edelstein CL. Cytokines in acute kidney injury (AKI). Clin Nephrol. 2011; 76:165–173.
21. Burket JS, Bartlett RH, Vander Hyde K, Chenoweth CE. Nosocomial infections in adult patients undergoing extracorporeal membrane oxygenation. Clin Infect Dis. 1999; 28:828–833.
22. Pappas PG, Kauffman CA, Andes D, Benjamin DK Jr, Calandra TF, Edwards JE Jr, Filler SG, Fisher JF, Kullberg BJ, Ostrosky-Zeichner L, et al. Clinical practice guidelines for the management of candidiasis: 2009 update by the Infectious Diseases Society of America. Clin Infect Dis. 2009; 48:503–535.
23. Doyle JS, Buising KL, Thursky KA, Worth LJ, Richards MJ. Epidemiology of infections acquired in intensive care units. Semin Respir Crit Care Med. 2011; 32:115–138.
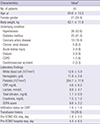



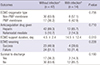





 PDF
PDF ePub
ePub Citation
Citation Print
Print



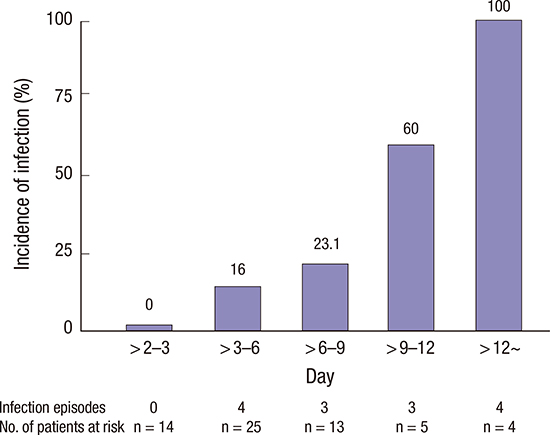

 XML Download
XML Download