Abstract
Objective
To evaluate the ultrasonography (US) features and clinical characteristics of columnar cell variant of papillary thyroid carcinoma (CCV-PTC) that can predict disease progression.
Materials and Methods
Six cases of CCV-PTC were identified via surgical pathology analysis at our institution from 1994 to 2016. The histological, architectural, and cytological features met the diagnostic criteria of CCV-PTC. We reviewed the US features and clinicopathological findings in the six cases.
Results
An indolent clinical course was observed in four young female patients aged 27–34 years (median: 32 years), while two older patients aged 55 years or 70 years had an aggressive clinical course. All patients underwent total thyroidectomy and radioiodine therapy. The indolent group included patients with T1 and nodal metastasis, where the disease was not observed during the follow-up period (range: 8–17 years). On the other hand, a larger tumor size (1.8 cm and 6.0 cm), gross extrathyroidal extension to the muscle and lymph node, and distant metastasis were observed in the aggressive group. In one male patient, recurrence occurred immediately after operation, and this patient died 4 years after the diagnosis of thyroid cancer. Based on US, the individuals from the indolent group had a smooth margin, except for one. Both cases in the aggressive group had a microlobulated margin.
Papillary thyroid carcinoma (PTC) is the most common malignancy in the thyroid gland, which accounts for 90% of all thyroid cancers (12). Several variants of PTC have been reported, and different histopathologic variants of PTC have varied clinical courses and prognosis (3). The columnar cell variant of papillary thyroid carcinoma (CCV-PTC) is a rare subtype, which accounts for 0.15–0.2% of all PTCs (4). The revised American Thyroid Association guidelines recently categorized the PTC variants according to their biological behavior as described in the literature, and CCV-PTC was classified as the aggressive type (5). Previous studies showed that CCV-PTC has a fast growth rate and a high incidence of recurrence, and this type of tumor is associated with local invasion and early lymph node (LN) metastasis (678). However, the prognosis of CCV-PTC remains controversial, because the encapsulated form has a more favorable outcome with indolent clinical process, which shows relatively slow growth and low incidence of recurrence or metastasis (91011). To the best of our knowledge, there were few reports about the imaging characteristics of CCV-PTC that can differ from other cell types in prognosis (12). Therefore, we evaluated the ultrasonography (US) features and clinical characteristics of CCV-PTC that can predict disease progression.
From 1994 to 2016, six patients were diagnosed with CCV-PTC via surgical pathology analysis in our institution. Data on the clinical characteristics and cytopathological results were obtained from the electric medical record database at our institution and retrospectively reviewed. This study was approved by our Institutional Review Board, and obtaining a written informed consent was waived due to the retrospective design of this study.
All six patients underwent preoperative thyroid US examination. The thyroid glands were scanned by experienced radiologists with HDI 5000 or iU22 scanners (Philips Medical Systems, Bothell, WA, USA) equipped with a commercially available 7- to 12-MHz linear transducer. All six preoperative US images were retrospectively reviewed by two board-certified radiologists with 18 years and 6 years of thyroid imaging experience, respectively. According to the Revised Korean Society of Thyroid Radiology Consensus Statement and Recommendations, the US features of the nodules were categorized based on the following: size (the maximal diameter of the nodule was documented), internal content (solid [no obvious cystic content], predominantly solid [≤ 50% of the cystic portion], predominantly cystic [> 50% of the cystic portion], and cystic [no obvious solid content]), echogenicity (markedly hypoechoic [hypoechoic relative to the anterior neck muscle], hypoechoic [hypoechoic relative to the thyroid parenchyma], isoechoic [similar echogenicity as the thyroid parenchyma], or hyperechoic [more echogenic relative to the thyroid parenchyma]), shape (round, ovoid, and irregular), orientation (parallel [the anteroposterior diameter of the nodule is equal to or less than its transverse or longitudinal diameter] or nonparallel [when the anteroposterior diameter of the nodule is larger than its transverse or longitudinal diameter]), margin (smooth, spiculated/microlobulated, or ill-defined), calcifications (microcalcifications [≤ 1 mm, brighter echo than the surrounding thyroid tissue], macrocalcifications [echogenic foci that is greater than 1 mm in size with posterior shadowing], and rim calcifications [peripheral curvilinear or eggshell calcification at the nodule margin]). Nodular vascularity was not routinely evaluated via color Doppler imaging throughout the study period. The radiologists made the final diagnosis for each nodule as “no nodule,” “benign,” “low suspicion,” “intermediate suspicion,” and “high suspicion” based on the Korean Thyroid Imaging Reporting and Data System (K-TIRADS) (13).
All six patients underwent preoperative US-guided fine needle aspiration (FNA) either in our institution (n = 3) or at other clinics (n = 3). The radiologists performed the preoperative US and US-guided FNA biopsy using a 23-gauge needle connected to a 2-mL disposable plastic syringe. FNA slides that were obtained from other clinics were reviewed by the pathology department of our institution. All six patients underwent total thyroidectomy. Prophylactic central compartment neck dissection for PTC surgery was performed in our institution before 2013. Modified lateral neck dissection was performed only when lateral neck LN metastasis was diagnosed at preoperative US and US-guided FNA. The final cytopathological diagnosis was evaluated by one of the seven pathologists, who was randomly selected based on their duty schedule. One specialized in thyroid pathology re-reviewed our cases to be CCV-PTC and agreed with their diagnosis.
The main clinical features and pathological results of the six patients with CCV-PTC are shown in detail in Table 1. The six patients aged 27–70 years (median age, 34 years; mean, 41.7 years) included in this study consisted of one male and five female patients. Clinical follow-up ranging from 1–17 years was (mean: 9 years) conducted for all six patients. Four cases were clinically indolent (cases 1–4), while two were aggressive (cases 5 and 6). Recurrence or distant metastasis (case 5 for brain metastasis, three years after diagnosis and case 6 for lung metastasis at the diagnosis) was observed in two patients during the follow-up period; these patients demonstrated recurrence immediately after surgery and died 4 years after diagnosis of thyroid cancer. During follow-up period, no metastasis and recurrence was detected in the other four patients (case 1–4). The median age was 32 years (range: 27–34 years) for the indolent group, and 66 years (55 years and 70 years) for the aggressive group. The mean tumor size was 1.2 cm (range: 0.4–2.0 cm) for the indolent group, and 3.9 cm (1.8 cm and 6.0 cm) for the aggressive group, respectively. Three out of the six patients underwent BRAFV600E mutation analysis (cases 2, 3, and 6). BRAFV600E mutation was observed in each patient from the indolent and aggressive groups; whereas one patient in the indolent group was negative for BRAFV600E mutation.
All six patients underwent preoperative US-guided FNA either in our institution (n = 3) or other clinics (n = 3). A specific variant could only be diagnosed in one out of six patients.
Four patients had CCV-PTCs in the right lobe, while two had CCV-PTCs in the left lobe. The median size of the nodule was 1.2 cm (range: 0.4–6.0 cm). Five patients underwent total thyroidectomy and central compartment neck dissection. One of the six patients underwent modified lateral neck dissection because lateral nodal metastasis was observed on preoperative US and FNA. Extrathyroidal extension to the muscle layer was observed in two patients (cases 5 and 6) and no microscopic extrathyroidal extension was reported in the other patients (cases 1–4).
Microscopically, CCV-PTC demonstrated microfollicles or elongated follicles of columnar cells with palisading oval nuclei and eosinophilic cytoplasm and minimal papillary nuclear features (Fig. 1).
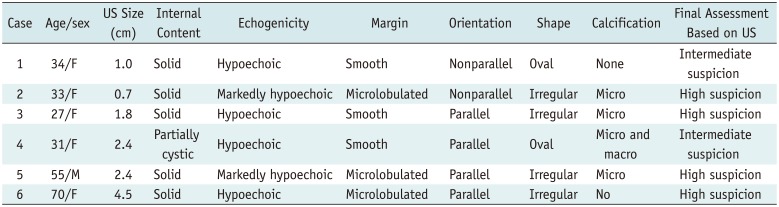
The US features of the six nodules are summarized in Table 2. The common US features of CCV-PTC were solid composition (n = 5, 83.3%), hypoechogenicity (n = 6, 100% [hypoechoic, n = 4], [markedly hypoechoic, n = 2]), and associated calcifications (n = 4, 66.7%, microcalcifications). The final diagnosis of the six nodules was intermediate suspicion (n = 2) or high suspicion (n = 4) based on K-TIRADS. Among the four patients in the indolent group, one (25%) had a nodule with a microlobulated margin, while 3 (75%) demonstrated smooth margins (Fig. 1); whereas both patients (100%) in aggressive group presented nodules with microlobulated margins (Fig. 2). Two nodules had parallel orientation, and two had a non-parallel orientation in the indolent group. On the other hand, all two patients presented with nodules that had parallel orientation in the aggressive group. Two patients from the indolent group had nodules with an irregular shape and two other nodules with an oval shape in indolent group. On the other hand, two patients in the aggressive group presented with nodules that are irregular in shape. In addition, the two nodules in the aggressive group showed infiltrative anterior margin abutting to anterior strap muscle which represented extrathyroidal extension. Color Doppler US was performed in four patients (cases 1, 3, 5, and 6), and the targeted Doppler US scan showed a variable vascular pattern with mild to marked vascularity.
Columnar cell variant of papillary thyroid carcinoma is a rare tumor that accounts for 0.15–0.2% of all PTCs, which was originally described as a hostile tumor by Evans in 1986 (47). The concept of CCV-PTC being a clinically aggressive tumor with a poor prognosis, was later challenged by several studies who reported more favorable outcomes in patients diagnosed with the encapsulated form of CCV-PTC (91014). However, these studies did not show the image features of CCV-PTC, which is clinically indolent. In the present study, only half of the patients in the indolent group had a tumor with the typical suspicious malignant feature (K-TIRADS 5), where the tumor size was relatively small and confined to the thyroid parenchyma.
In 2011, Chen et al. (15) reviewed several previous studies and presented 48 patients of CCV-PTC, of which 20 were clinically indolent (18 women and 2 men; mean age, 44.9 years), and 23 cases where the tumors were considered as aggressive (10 women and 13 men; mean age, 55.6 years). The size of the indolent tumors ranged from 0.9–8.0 cm (mean size: 3.6 cm; median, 3.8 cm), and that of the aggressive tumors ranged from 0.6–10.0 cm (mean size, 6.0 cm; median, 6.3 cm). Of the cases with clinical follow-up, almost all the patients with indolent tumors (18 out of 19) were alive or free from the disease for 9 months to 22 years after diagnosis. Of the 20 patients with aggressive tumors, 13 died from disease approximately 7–126 months after diagnosis. Also, the extrathyroidal extension of clinically indolent CCV-PTC were not reported while most of clinically aggressive CCV-PTC showed extrathyroidal extension, accounting for 67–100% (111516). Similarly to the previous studies, the four indolent tumors in our study were small (mean size: 1.2 cm), encapsulated, confined to the thyroid gland, and present in younger individuals (range: 27–34 years). Recurrence was not observed in the female patients during the clinical follow-up. Most individuals in the indolent group had nodules with a smooth margin based on US. On the other hand, two patients had aggressive tumors that were larger (1.8 cm and 6.0 cm) than the indolent tumors, extrathyroidal extension, metastasis to LNs and distant organs, affected older patients (55 years and 70 years) and died of their disease, 4 years after diagnosis. A previous report insisted that CCV-PTC is a unique histologic subtype but not a unique clinical type of tumor and treatment of patients with these tumors should be based on the clinical stage and not on the histomorphologic appearance (11). The results of our study suggest that the CCV-PTC has two distinct clinical types, one of which is more fatal than the similar stage of conventional PTC. Because the case number of our series was too small to draw a conclusion, further investigation including US features is necessary to distinguish aggressive CCV-PTC from indolent CCV-PTC and conventional PTC for risk stratification.
To the best our knowledge, studies on the imaging characteristics of CCV-PTC have been limited. US may show hypoechoic nodules with or without calcifications and with at least more than one typical malignant sonographic feature (12). Compared with previous studies, most patients with CCV-PTC masses in our cases had solid and hypoechoic nodules (n = 5), mostly accompanied by microcalcifications (n = 4). Consequently, the final diagnosis was intermediate suspicion (n = 2) or high suspicion (n = 4) nodules according to the K-TIRADS. Based on US, the indolent tumors had a smooth margin, except for one, when compared to the tumors of two patients in the aggressive group who had microlobulated margins. Furthermore, the two tumors in the aggressive group presented infiltrative anterior margin abutting to anterior strap muscle which was suspicious for extrathyroidal extension. Aggressive tumors were more frequently classified as high suspicion by US than the indolent tumors. In the current study, there are too few cases to find US findings that can distinguish conventional PTC from CCV-PTC on the preoperative status. However, if surgical pathology reveals CCV-PTC in the postoperative status and the US finding is consistent with the indolent type, the further management with the aggressive protocol can be avoided.
Only one of the six cases was recognized as CCV-PTC based on cytology. Based on histology, CCV-PTC is defined as papillae or gland-like structures lined by columnar cells that have prominent nuclear stratification. The accurate diagnosis of CCV-PTC is difficult upon FNA cytology, because the tumor cells in CCV-PTC lack the nuclear features of the classic pattern of papillary carcinoma, which show nuclear grooving and intranuclear pseudo-inclusions (1718). The most useful features in the differentiation of CCV-PTC from other PTC is the presence of columnar cells that exhibit the stratification of nuclei over the papillaroid fragments (19).
Our study had several limitations. First, because of the low incidence of CCV-PTC, the sample size of this study was small. Second, this study was retrospective in nature. Thus, potential selection bias may have affected the results, and not all parameters of the US features had been fully evaluated, such as the color Doppler US.
In conclusion, favorable prognosis in CCV-PTC is observed in young patients with T1 staging and demonstrates a smooth margin at US. These US findings might help exclude the same treatment as in the aggressive type in the indolent type of CCV-PTC.
References
1. Hundahl SA, Fleming ID, Fremgen AM, Menck HR. A National Cancer Data Base report on 53,856 cases of thyroid carcinoma treated in the U.S., 1985-1995 [see commetns]. Cancer. 1998; 83:2638–2648. PMID: 9874472.
2. Papp S, Asa SL. When thyroid carcinoma goes bad: a morphological and molecular analysis. Head Neck Pathol. 2015; 9:16–23. PMID: 25804379.

3. Lee JH, Shin JH, Lee HW, Oh YL, Hahn SY, Ko EY. Sonographic and cytopathologic correlation of papillary thyroid carcinoma variants. J Ultrasound Med. 2015; 34:1–15.

4. Sywak M, Pasieka JL, Ogilvie T. A review of thyroid cancer with intermediate differentiation. J Surg Oncol. 2004; 86:44–54. PMID: 15048680.

5. Haugen BR, Alexander EK, Bible KC, Doherty GM, Mandel SJ, Nikiforov YE, et al. 2015 American Thyroid Association management guidelines for adult patients with thyroid nodules and differentiated thyroid cancer: the American Thyroid Association guidelines task force on thyroid nodules and differentiated thyroid cancer. Thyroid. 2016; 26:1–133. PMID: 26462967.

6. Berends D, Mouthaan PJ. Columnar-cell carcinoma of the thyroid. Histopathology. 1992; 20:360–362. PMID: 1577416.

7. Evans HL. Columnar-cell carcinoma of the thyroid. A report of two cases of an aggressive variant of thyroid carcinoma. Am J Clin Pathol. 1986; 85:77–78. PMID: 3940424.

8. Gaertner EM, Davidson M, Wenig BM. The columnar cell variant of thyroid papillary carcinoma. Case report and discussion of an unusually aggressive thyroid papillary carcinoma. Am J Surg Pathol. 1995; 19:940–947. PMID: 7611541.

9. Evans HL. Encapsulated columnar-cell neoplasms of the thyroid. A report of four cases suggesting a favorable prognosis. Am J Surg Pathol. 1996; 20:1205–1211. PMID: 8827026.
10. Ferreiro JA, Hay ID, Lloyd RV. Columnar cell carcinoma of the thyroid: report of three additional cases. Hum Pathol. 1996; 27:1156–1160. PMID: 8912824.

11. Wenig BM, Thompson LD, Adair CF, Shmookler B, Heffess CS. Thyroid papillary carcinoma of columnar cell type: a clinicopathologic study of 16 cases. Cancer. 1998; 82:740–753. PMID: 9477108.
12. Shin JH. Ultrasonographic imaging of papillary thyroid carcinoma variants. Ultrasonography. 2017; 36:103–110. PMID: 28222584.

13. Shin JH, Baek JH, Chung J, Ha EJ, Kim JH, Lee YH, et al. Ultrasonography diagnosis and imaging-based management of thyroid nodules: revised Korean Society of Thyroid Radiology consensus statement and recommendations. Korean J Radiol. 2016; 17:370–395. PMID: 27134526.

14. Huang WT, Yang SF, Wang SL, Chan HM, Chai CY. Encapsulated columnar-cell carcinoma of the thyroid: a case report. Kaohsiung J Med Sci. 2005; 21:241–244. PMID: 15960072.

15. Chen JH, Faquin WC, Lloyd RV, Nosé V. Clinicopathological and molecular characterization of nine cases of columnar cell variant of papillary thyroid carcinoma. Mod Pathol. 2011; 24:739–749. PMID: 21358618.

16. Sujoy V, Pinto A, Nosś V. Columnar cell variant of papillary thyroid carcinoma: a study of 10 cases with emphasis on CDX2 expression. Thyroid. 2013; 23:714–719. PMID: 23488912.

17. Verma R, Paul P. Columnar cell variant of papillary thyroid carcinoma: a diagnostic dilemma in fine-needle aspiration cytology. Diagn Cytopathol. 2016; 44:816–819. PMID: 27279270.

18. Ylagan LR, Dehner LP, Huettner PC, Lu D. Columnar cell variant of papillary thyroid carcinoma. Report of a case with cytologic findings. Acta Cytol. 2004; 48:73–77. PMID: 14969185.
19. Bongiovanni M, Mermod M, Canberk S, Saglietti C, Sykiotis GP, Pusztaszeri M, et al. Columnar cell variant of papillary thyroid carcinoma: cytomorphological characteristics of 11 cases with histological correlation and literature review. Cancer Cytopathol. 2017; 125:389–397. PMID: 28374549.

Fig. 1
Case 3: 27-year-old woman with CCV-PTC diagnosed with fine-needle aspiration cytology.
Transverse (A) and longitudinal (B) US shows 1.8-cm hypoechoic solid nodule (arrows) with oval shape, smooth margin, and microcalcification confined to right thyroid gland. (C) Note microfollicles or elongated follicles of columnar cells (arrows) with palisading oval nuclei and eosinophilic cytoplasm, and minimal papillary nuclear features, Hematoxylin & eosin (× 400). CCV-PTC = columnar cell variant of papillary thyroid carcinoma, US = ultrasonography
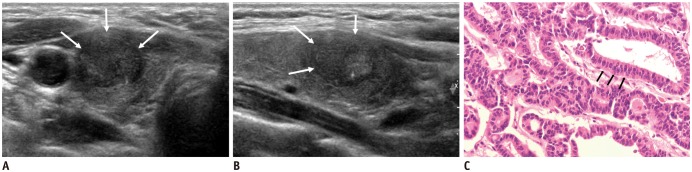
Fig. 2
Case 5: CCV-PTC in 55-year-old man.
Transverse (A) and longitudinal (B) US shows 2.4-cm hypoechoic solid nodule (arrows) with irregular shape, microlobulated margin (arrowheads), and microcalcification (thin arrows) in right thyroid gland. Nodule presented infiltrative anterior margin abutting to anterior strap muscle which was suspicious for extrathyroidal extension. Finally, gross extrathyroidal extension on pathology and surgical report was present. He was included in aggressive group and died from disease 4 years after diagnosis.
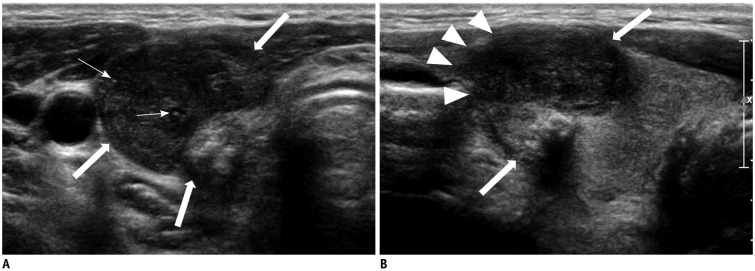
Table 1
Clinicopathological Features of Six Patients with CCV-PTC
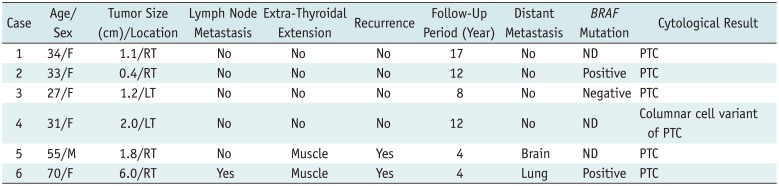
Table 2
Ultrasonographic Features of Six CCVs-PTC




 PDF
PDF ePub
ePub Citation
Citation Print
Print


 XML Download
XML Download