Abstract
Purpose
Subacute sclerosing panencephalitis (SSPE) is a neurodegerative disease due to persistent measles virus infection. We investigated the role of programmed death-1 (PD-1) molecule which is related with chronic viral infection in developing SSPE in mouse.
Methods
We adopt the PD-1-/-, PD-1-/+, and wild type BALB/c 3 week old mice to make an animal model of SSPE by injecting measles virus (SSPE strain) intraventricularly. Three months after infusion of virus, the mice were sacrificed and examined if the typical pathologic lesions had been progressed. The sera were collected from each group of mice and the serum level of IL-21 was measured with ELISA kit.
Figures and Tables
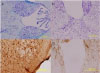
Fig. 1
Changes of brain parenchyma by SSPE virus in 3 month-old wild type BALB/c. (A) No change was shown in wild mice which was not injected with virus (×200, Nissl staining). (B) Inflammatory changes were observed in the same age of wild mice which was injected with SSPE virus (×400, Nissl staining).

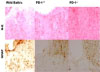
Fig. 2
Comparison of lesions according to PD-1 status. (A) Wild type mouse (×200, Nissl staining) (B) Hetero type mouse (×200, Nissl staining). The inflammation in the myelin portion is more serious in wild type mouse than in heterotype. (C) Wild type mouse (×200, GFAP staining). (D) KO mouse (×200, GFAP staining). The astrocytes are proliferated more in wild type mouse than in KO mouse.
References
1. van den Ent MM, Brown DW, Hoekstra EJ, Christie A, Cochi SL. Measles mortality reduction contributes substantially to reduction of all cause mortality among children less than five years of age, 1990-2008. J Infect Dis. 2011; 204:Suppl 1. S18–S23.

2. Oldstone MB, Dales S, Tishon A, Lewicki H, Martin L. A role for dual viral hits in causation of subacute sclerosing panencephalitis. J Exp Med. 2005; 202:1185–1190.

4. Schneider-Schaulies S, ter Meulen V. Pathogenic aspects of measles virus infections. Arch Virol Suppl. 1999; 15:139–158.

5. Gascon GG. Randomized treatment study of inosiplex versus combined inosiplex and intraventrcular interferon-alpha in subacute sclerosing panencephalitis (SSPE): International multicenter study. J child Neurol. 2003; 18:819–827.

6. Eroglu E, Gokcil Z, Bek S, Ulas UH, Ozdag MF, Odabasi Z. Long-term follow-up of patients with adult-onset subacute sclerosing panencephalitis. J Neurol Sci. 2008; 275:113–116.

7. Cathomen T, Mrkic B, Spehner D, Drillien R, Naef R, Pavlovic J, et al. A matrix-less measles virus is infectious and elicits extensive cell fusion: consequences for propagation in the brain. EMBO J. 1998; 17:3899–3908.

8. Moulin E, Beal V, Jeantet D, Horvat B, Wild TF, Waku-Kouomou D. Molecular characterization of measles virus strains causing subacute sclerosing panencephalitis in France in 1977 and 2007. J Med Virol. 2011; 83:1614–1623.

9. Forcic D, Baricevic M, Zgorelec R, Kruzic V, Kaic B, Marina BM, et al. Detection and characterization of measles virus strains in cases of subacute sclerosing panencephalitis in Croatia. Virus Res. 2004; 99:51–56.

10. Wong TC, Ayata M, Ueda S, Hirano A. Role of biased hypermutation in evolution of subacute sclerosing panencephalitis virus from progenitor acute measles virus. J Virol. 1991; 65:2191–2199.

11. Ha SJ, Mueller SN, Wherry EJ, Barber DL, Aubert RD, Sharpe AH, et al. Enhancing therapeutic vaccination by blocking PD-1-mediated inhibitory signals during chronic infection. J Exp Med. 2008; 205:543–555.

12. Macatangay BJ, Rinaldo CR. PD-1 blockade: A promising immunotherapy for HIV? Cellscience. 2009; 5:61–65.
13. Reuter D, Schneider-Schaulies J. Measles virus infection of the CNS: human disease, animal models, and approaches to therapy. Med Microbiol Immunol. 2010; 199:261–271.

14. Oldstone MB. Modeling subacute sclerosing panencephalitis in a transgenic mouse system: uncoding pathogenesis of disease and illuminating components of immune control. Curr Top Microbiol Immunol. 2009; 330:31–54.

15. Griffin DE, Lin WH, Pan CH. Measles virus, immune control, and persistence. FEMS Microbiol Rev. 2012; 36:649–662.

16. Tucker WG, Andrew Paskauskas R. The MSMV hypothesis: measles virus and multiple sclerosis, etiology and treatment. Med Hypotheses. 2008; 71:682–689.

17. Zhu B, Guleria I, Khosroshahi A, Chitnis T, Imitola J, Azuma M, et al. Differential role of programmed deathligand 1 [corrected] and programmed death-ligand 2 [corrected] in regulating the susceptibility and chronic progression of experimental autoimmune encephalomyelitis. J Immunol. 2006; 176:3480–3489.

18. Salama AD, Chitnis T, Imitola J, Ansari MJ, Akiba H, Tushima F, et al. Critical role of the programmed death-1 (PD-1) pathway in regulation of experimental autoimmune encephalomyelitis. J Exp Med. 2003; 198:71–78.

19. Kroner A, Schwab N, Ip CW, Ortler S, Gobel K, Nave KA, et al. Accelerated course of experimental autoimmune encephalomyelitis in PD-1-deficient central nervous system myelin mutants. Am J Pathol. 2009; 174:2290–2299.

20. Ishizaki Y, Takemoto M, Kira R, Kusuhara K, Torisu H, Sakai Y, et al. Association of toll-like receptor 3 gene polymorphism with subacute sclerosing panencephalitis. J Neurovirol. 2008; 14:486–491.

21. Lafon M, Megret F, Meuth SG, Simon O, Velandia Romero ML, Lafage M, et al. Detrimental contribution of the immuno-inhibitor B7-H1 to rabies virus encephalitis. J Immunol. 2008; 180:7506–7515.





 PDF
PDF ePub
ePub Citation
Citation Print
Print


 XML Download
XML Download