Abstract
PURPOSE
To evaluate the cytotoxicity of temporary luting cements on bovine dental pulp-derived cells (bDPCs).
MATERIALS AND METHODS
Four different temporary cements were tested: Rely X Temp E (3M ESPE), Ultratemp (Ultradent), GC Fuji Temp (GC), and Rely X Temp NE (3M ESPE). The materials were prepared as discs and incubated in Dulbecco's modified eagle's culture medium (DMEM) for 72 hours according to ISO 10993-5. A real-time cell analyzer was used to determine cell vitality. After seeding 200 µL of the cell suspensions into the wells of a 96-well plate, the bDPCs were cured with bioactive components released by the test materials and observed every 15 minutes for 98 hours. One-way ANOVA and Tukey-Kramer tests were used to analyze the results of the proliferation experiments.
RESULTS
All tested temporary cements showed significant decreases in the bDPCs index. Rely X Temp E, GC Fuji Temp, and Rely X Temp NE were severely toxic at both time points (24 and 72 hours) (P<.001). When the cells were exposed to media by Ultratemp, the cell viability was similar to that of the control at 24 hours (P>.05); however, the cell viability was significantly reduced at 72 hours (P<.001). Light and scanning electron microscopy examination confirmed these results.
Temporary cements are used for luting provisional indirect restorations including inlays, onlays, full crowns, and fixed partial dentures (FPDs), as well as for temporarily luting definitive restorations of the same types.1,2,3 Before the bonding of the final restoration, temporary cements are required to veneer the freshly prepared tooth and to guard the pulp from peripheral stressors such as thermal, mechanical and noxious microbial effects for the required time period, as well as to maintain adequate masticatory function, phonetics and esthetics.4,5 These cements cap the pulp, and the teeth should be observed for signs of possible irreversible pulp damage until placement of the final restoration.6
Because the temporary cement is located very close to the pulpal tissue and maintains prolonged contact with the freshly cut dentin-pulpal complex, its impact on pulpal cells is of great interest, especially when the dentin is thin or pulp exposure is noted during tooth preparation or caries removal.7 There is a critical importance for freshly opened dentinal tubules after tooth preparation due to pulpal vulnerability.8 Toxic elements released from these cements may cause a reaction in adjacent tissues, such as pulp, gingiva, alveolar bone or mucosa. Thus, the biocompatibility of temporary cements is required for clinical success. The toxicity and biocompatibility of permanent traditional and resin luting cements have been widely studied, and prior findings show varying degrees of biological effects.7,8,9 These studies reported that the tested resin-based cements significantly decreased cell vitality.7,9 However, little information is available in the prosthodontic literature concerning the biocompatibility of temporary cements.
Although the developments and improvements in temporary cements are satisfying, their biocompatibility is an important question for dentistry. In our present study, we investigated the cytotoxicity of four different temporary cements on dental pulp-derived cells (bDPCs) with realtime and continuous monitoring of cell vitality.
Four different temporary cements were tested in this experiment: Rely X Temp E (3M ESPE, St. Paul, MN, USA), Ultratemp (Ultradent Products, South Jordan, UT, USA), GC Fuji Temp (GC America Inc, Alsip, IL, USA), Rely X Temp NE (3M ESPE, St. Paul, MN, USA). Material details and compositions are shown in Table 1. Samples were manufactured according to the recommendations using standard Teflon plates (5 × 2 mm diameter and depth) under disinfected conditions. Ten samples were used from each group for testing.
The bPDCs (the cells were named SVNeo3 cells by the group) were kindly provided from Prof G. Schmalz (Regensburg University)7 and were cultured in α-MEM supplemented with 20% FBS, penicillin (150 IU/mL), gentamicin (0.1 mg/mL), and streptomycin (150 µg/mL) at 37℃ and 5% CO2. Cells within passages 19 to 23 were used.
The specimens (Rely X Temp E, Ultratemp, and Rely X Temp NE) were submerged in seven mL of culture medium for one day at 37℃ to remove residual monomer or cytotoxic substances. The culture medium containing the material extracts was filtered to use on the cell cultures. The test designs for this study were completed following ISO 10993-5.10 Control experiments contained only medium.
The system of xCELLigence (Roche Applied Science, Mannheim, Germany) consists of four main modules: an impedance-based real time cell analyzer (RTCA), an RTCA single plate, an RTCA computer, and a 96-well plate. The RTCA single plate was positioned in a tissue-culture incubator. The electronic impedance of the sensor electrodes was measured to allow observing and revealing of physiologic changes in the cells. The impedance measured between electrodes in each well depends on electrode geometry, ion concentration in the well, and whether the cells are attached to the electrodes. In the absence of cells, electrode impedance is mainly determined by the ion environment at the electrode-solution interface and in the bulk solution. In the presence of cells, cells attached to the electrode sensor surfaces act as insulators and thereby alter the local ion environment at the electrode-solution interface, leading to increased impedance.11,12
The tested material samples were incubated in DMEM culture medium for 72 hours according to ISO 10993-5. The bPDCs were maintained with DMEM containing 10% fetal bovine serum. The xCELLigence system was used to assess cell survival. After seeding 200 mL of the cell suspension into each wells (7,500 cells/well) of the 96-well plate, the bDPCs were preserved with bioactive components released by the luting cement materials and were monitored every 15 minutes for 72 hours. Control samples received only medium.
Morphologic alterations of the bDPCs were observed directly using an inverted microscope (10x; TS100 Nikon Eclipse, Tokyo, Japan) and photographed by a camera (Nikon Eclipse, Tokyo, Japan) at 30 minutes and 2 hours.
To evaluate the morphology and adhesion of the bDPCs (100,000 cells (25 µL)/resin discs), cells were seeded on the luting resin cements, which were placed on tissue culture inserts. Cellular adhesion was assessed with scanning electron microscopy (SEM) on day 3. For SEM evaluation, cells were fixed on the resin cements for 15 minutes with 2.5% glutaraldehyde in 0.01 M PBS. After removal from the inserts, the specimens were dried and sputtercoated with gold. The surfaces of the resin cements and the adhesion profile of the bDPCs were photographed with an SEM (Zeiss EVO ® LS 10, Brock & Michelsen, Denmark).
Data were represented as the mean (mmol/L) ± SD. One-way analysis of variance (ANOVA) and Tukey-Kramer multiple comparison tests were used to analyze the results from the proliferation experiments. A P value <.05 was considered to be statistically significant at α=.05.
All tested temporary cements showed significant decreases in the bDPCs index. Rely X Temp E, GC Fuji Temp, and Rely X Temp NE were severely toxic at both time points (24 and 72 hours) (P<.001). When the cells were exposed to media with bioactive components released by Ultratemp, the cell viability was similar to that of the control at 24 hours (P>0.05); however, the cell viability was reduced at 72 hours (P<.001) (Fig. 1, Table 2).
When viewed with light microscopy, the bPDCs were elongated in appearance. While exposure to Rely X Temp E and Ultratemp led to expansion of the intercellular gap even after 30 and 120 minutes, the cells' spindle-like appearance was preserved. Cell density decreased in the tested groups compared to that of the control group. Light microscopy images confirmed the real-time cell analyzer results. As the toxicity increased, the cells had a rounded shape, indicating that they were no longer alive, especially the cells in the GC Fuji Temp and Rely X Temp NE groups (Fig. 2).
In the SEM examination, the cells exhibited ovoid to rounded morphology with cytoplasmic extensions adapting to the tested temporary cements. However, the surface characteristics of Ultratemp seemed different when compared to the other materials; the shapes of cells attached to Ultratemp appear more preserved when compared to cells on Rely X Temp NE and Rely X Temp E (Fig. 3). The bPDCs had a rounded form, especially the bPDCs in the GC Fuji Temp and Rely X Temp NE groups, demonstrating that they were datival (Fig. 2).
The research obviously demonstrated that the Rely X Temp E, GC Fuji Temp, and Rely X Temp NE were more toxic than Ultratemp. In addition, Ultratemp was also cytotoxic materials when compared to the control at 72 hours.
Using cell culture to investigate dental materials has several benefits: it is relatively easy to perform, reproducible, cost effective, and accurately controlled.12,13,14,15,16 To investigate the biocompatibility of temporary luting cements, dental pulp-derived cells (bDPCs) were used, and this study first performed an evaluation of the cytotoxicity of several temporary luting cements using real-time and continuous monitoring of cell vitality. Using cell culture to investigate dental materials has several benefits: it is relatively easy to perform, reproducible, cost effective, and accurately controlled.12,13,14,15,16 Real-time and uninterrupted observation permits label-free evaluation of cell proliferation, vitality, and toxicity. Compared to classical endpoint cell-based assays, dynamic observation of cellular conditions, such as cell adhesion, increase, proliferation, growth, and apoptosis provides a great advantage of the real-time design for optimization of cell concentrations and conditions for in vitro assays before and during experiments.11,12 The real-time test method proved to be useful to estimate cell densities in small culture volumes. Cell cultivation in small culture volumes and sensitive evaluation with real-time tests permits screening and testing of many different substances and concentrations to determine cytotoxicity.11,12 For these reasons, we chose to use the real-time xCELLigence test procedure.
Primary pulp cells are very similar to genuine tissue and almost have non-changed metabolism. So, in laboratory condition, primary pulp cells can be a superior mimic. However, separation of primary cells from pulp requires much effort and is time-consuming, and the amount of resulting cells is often very small. Moreover, primary pulp cells have inadequate potential to divide and reach a non-proliferative status. In last years, to solve this problem, an immortalized bovine dental papilla-derived cell line was developed by transfection with coding sequences of Simian Virus 40 (SV40) large T-antigen.17,18 The major problem seems to be that this cell lines may be not the same from the original tissue. However, this cell lines were demonstrated to have the same biologic features as primary dental pulp cells. Consequently, it was suggested that this cell lines would be the ideal selections for biocompatibility investigations of dental materials.16 Primary pulp cells are closely related to their original tissue and have a nearly unchanged metabolic state relative to this tissue. Thus, in vivo conditions may be better mimicked by conditions using primary pulp cells. And, they have also exhibited a high proliferation rate with the capability of sub-culturing for a high number of passages.
Zinc oxide-eugenol cements (ZOE) are widely used for the temporary filling and luting of cast restorations. The biological properties of ZOE materials include desirable effects such as pain elimination, but they also provoke unwanted reactions. In the present study, the biocompatibility of commercially available ZOE and zinc oxide noneugenol (ZONE) temporary cements were investigated. Both ZOE and ZONE cements showed varying degrees of cytotoxicity, where the results from cultured cells were in agreement with those from many previous studies.19,20,21 Zinc oxide-eugenol is an important component of several provisional cements because of its bacteriostatic or bactericidal effects, low cost, easy clinical manipulation, and good sealing properties.2 On the other hand, the toxicity of ZOE and ZONE cements has been demonstrated to be produced by the components of each material, such as the eugenol released from ZOE cements and zinc ion, which is released from both ZOE and ZONE cements.22 Hensten-Pettersen and Helgeland23 reported obvious cytotoxic effects of eugenol in four different test designs even 24 h after mixing. Direct pulp capping using ZOE cements on exposed vital pulp will lead to a severe inflammatory response and pulp necrosis.24
Ülker et al.25 reported that zinc oxide-containing temporary cement is not cytotoxic to bovine pulp-derived cells when tested using MTT method, even if dentin barrier does not exist. The present study demonstrated that Ultratemp, which contains zinc oxide, was cytotoxic to bDPCs. The test design and investigation conditions like as xCELLigence system and bDPCs cultures of present study are different from Ülker et al.25 However, Kwon et al.21 studied cytotoxicity of commercially available ZOE and ZONE cements by MTT test. They demonstrated that both ZOE and ZONE cements showed varying degrees of cytotoxicity in accordance with our data. These conflicted results may explain on test design, cell cultures differences, tested materials substance differences. And, they concluded that cytotoxicity evaluation for ZOE and ZONE cements were clearly different between animal-based cell line and humanbased primary cells. The results may depend on these differences.
Rely X Temp NE includes ZO and resin. Mixtures of ZO and resin are used as components of periodontal pastes, cements and root canal sealers. These materials also showed cytotoxic effects in this study. Other ingredients of both GC Fuji Temp and Rely X Temp NE are acrylic acid and polyacrylic acid, and Kurata et al.26 reported that fibroblast proliferation decreased upon exposure to acrylic acid as the concentration of acid increased. Additionally, Sunzel et al.27 reported that high amounts of resin mixture and pure resin acids were strongly associated with toxicity. GC Fuji Temp and Rely X Temp NE were demonstrated to be toxic to bDPCs cells in the present study as well.
Clinically, the thickness of the dentin layer between the pulp and temporary cement material is very important with regard to cytotoxicity. To mimic the clinical situation for temporary cements, artificial pulp chamber methods introducing dentin as a wall between test materials and pulp cells can be used in future studies. The dentin layer can be protective of pulpal tissue against the cytotoxic effects of temporary cements.28 The present study demonstrated that except for Ultratemp, the tested temporary luting cements were cytotoxic to bDPCs cells. Hanks et al.29 demonstrated that a layer of dentin can be used as a partial perfusion/diffusion wall for permeability testing. Dentin may serve as a diffusion and adsorption wall, thus decreasing the concentration of toxic elements that reach the pulpal tissue and possibly cause toxic reactions.30
In conclusion, in the present study, it was demonstrated that there was a difference among the tested materials when evaluating their cytotoxicity results using bDPCs cells. Future studies comparing the cytotoxicity results from different test methods or designs in correlation with in vivo or clinical biocompatibility methods will be useful when considering test methods to obtain clinically relevant results.
It appears that the tested temporary cements may be capable of causing pulpal damage. Dentists should therefore carefully consider the use of temporary cements on freshly cut dentin preparations.
Figures and Tables
Fig. 1
Dynamic monitoring of cultured dental pulp-derived cells (bDPCs) adhesion and cell proliferation.

Fig. 2
Light microscope images of cultured dental pulp-derived cells (bDPCs) at 30 and 120 minutes. Control, a: Rely X Temp E, b: Ultratemp, c: GC Fuji Temp, d: Rely X Temp NE.
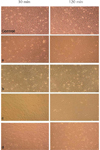
Fig. 3
Scanning electron microscope images of cultured dental pulp-derived cells (bDPCs). a: Rely X Temp E, b: Ultratemp, c: GC Fuji Temp, d: Rely X Temp NE.

Table 1
Temporary cements used in this study

Table 2
Cell index by real time cell analysis and comparison of 24 and 72 hours with ANOVA and Tukey Krammer multiple comparison tests

References
1. Pashley EL, Tao L, Pashley DH. The sealing properties of temporary filling materials. J Prosthet Dent. 1988; 60:292–297.
2. Rosenstiel SF, Land MF, Fujimoto J. Contemporary Fixed Prosthodontics. In : Rosenstiel SF, editor. Luting agents and cementation procedures. St. Louis; Missouri: Mosby Elsevier;2006. p. 909–925.
3. Olin PS, Rudney JD, Hill EM. Retentive strength of six temporary dental cements. Quintessence Int. 1990; 21:197–200.
4. Ribeiro JC, Coelho PG, Janal MN, Silva NR, Monteiro AJ, Fernandes CA. The influence of temporary cements on dental adhesive systems for luting cementation. J Dent. 2011; 39:255–262.
5. Paul SJ, Schärer P. Effect of provisional cements on the bond strength of various adhesive bonding systems on dentine. J Oral Rehabil. 1997; 24:8–14.
6. Albers HF. Tooth-colored restoratives: Principles and techniques. In : Albers HF, editor. Diagnosis. London: BC Decker;2002. p. 23–24.
7. Ülker HE, Hiller KA, Schweikl H, Seidenader C, Sengun A, Schmalz G. Human and bovine pulp-derived cell reactions to dental resin cements. Clin Oral Investig. 2012; 16:1571–1578.
8. Kong N, Jiang T, Zhou Z, Fu J. Cytotoxicity of polymerized resin cements on human dental pulp cells in vitro. Dent Mater. 2009; 25:1371–1375.
9. Malkoc S, Corekci B, Botsali HE, Yalçin M, Sengun A. Cytotoxic effects of resin-modified orthodontic band adhesives. Are they safe. Angle Orthod. 2010; 80:890–895.
10. ISO 10993-5. Biological evaluation of medical devices - Part 5: Tests for in vitro cytotoxicity. Geneva; Switzerland: International Standards Organization (ISO);2009.
11. Urcan E, Haertel U, Styllou M, Hickel R, Scherthan H, Reichl FX. Real-time xCELLigence impedance analysis of the cytotoxicity of dental composite components on human gingival fibroblasts. Dent Mater. 2010; 26:51–58.
12. Malkoç S, Öztürk F, Çörekçi B, Bozkurt BS, Hakki SS. Realtime cell analysis of the cytotoxicity of orthodontic mini-implants on human gingival fibroblasts and mouse osteoblasts. Am J Orthod Dentofacial Orthop. 2012; 141:419–426.
13. Schmalz G. Use of cell cultures for toxicity testing of dental materials-advantages and limitations. J Dent. 1994; 22:S6–S11.
14. Costa MT, Lenza MA, Gosch CS, Costa I, Ribeiro-Dias F. In vitro evaluation of corrosion and cytotoxicity of orthodontic brackets. J Dent Res. 2007; 86:441–445.
15. Koulaouzidou EA, Helvatjoglu-Antoniades M, Palaghias G, Karanika-Kouma A, Antoniades D. Cytotoxicity of dental adhesives in vitro. Eur J Dent. 2009; 3:3–9.
16. Thonemann B, Schmalz G. Immortalization of bovine dental papilla cells with simian virus 40 large t antigen. Arch Oral Biol. 2000; 45:857–869.
17. Sengün A, Yalçın M, Ülker HE, Öztürk B, Hakkı SS. Cytotoxicity evaluation of dentin bonding agents by dentin barrier test on 3-dimensional pulp cells. Oral Surg Oral Med Oral Pathol Oral Radiol Endod. 2011; 112:e83–e88.
18. Galler KM, Schweikl H, Thonemann B, D›Souza RN, Schmalz G. Human pulp-derived cells immortalized with Simian Virus 40 T-antigen. Eur J Oral Sci. 2006; 114:138–146.
19. Koulaouzidou EA, Papazisis KT, Economides NA, Beltes P, Kortsaris AH. Antiproliferative effect of mineral trioxide aggregate, zinc oxide-eugenol cement, and glass-ionomer cement against three fibroblastic cell lines. J Endod. 2005; 31:44–46.
20. Torabinejad M, Hong CU, Pitt Ford TR, Kettering JD. Cytotoxicity of four root end filling materials. J Endod. 1995; 21:489–492.
21. Kwon JS, Illeperuma RP, Kim J, Kim KM, Kim KN. Cytotoxicity evaluation of zinc oxide-eugenol and non-eugenol cements using different fibroblast cell lines. Acta Odontol Scand. 2014; 72:64–70.
22. Schmalz G, Rotgans J. An in vitro study on the toxicity of an odour-free and flavourless zinc oxide and eugenol cement. Ned Tijdschr Tandheelkd. 1979; 86:85–88.
23. Hensten-Pettersen A, Helgeland K. Evaluation of biologic effects of dental materials using four different cell culture techniques. Scand J Dent Res. 1977; 85:291–296.
24. Sela J, Ulmansky M. Reaction of normal and inflamed dental pulp to Calxyl and zinc oxide and eugenol in rats. Oral Surg Oral Med Oral Pathol. 1970; 30:425–430.
25. Ülker HE, Ülker M, Gümüş HÖ, Yalçın M, Şengün A. Cytotoxicity testing of temporary luting cements with twoand three-dimensional cultures of bovine dental pulp-derived cells. Biomed Res Int. 2013; 2013:910459.
26. Kurata S, Morishita K, Kawase T, Umemoto K. Cytotoxic efeffects of acrylic acid, methacrylic acid, their corresponding saturated carboxylic acids, HEMA, and hydroquinone on fibroblasts derived from human pulp. Dent Mater J. 2012; 31:219–225.
27. Sunzel B, Söderberg TA, Johansson A, Hallmans G, Gref R. The protective effect of zinc on rosin and resin acid toxicity in human polymorphonuclear leukocytes and human gingival fibroblasts in vitro. J Biomed Mater Res. 1997; 37:20–28.
28. Hume WR. Influence of dentine on the pulpward release of eugenol or acids from restorative materials. J Oral Rehabil. 1994; 21:469–473.
29. Hanks CT, Wataha JC, Parsell RR, Strawn SE, Fat JC. Permeability of biological and synthetic molecules through dentine. J Oral Rehabil. 1994; 21:475–487.
30. Schmalz G, Hoffmann M, Weis K, Schweikl H. Influence of albumin and collagen on the cell mortality evoked by zinc oxide-eugenol in vitro. J Endod. 2000; 26:284–287.




 PDF
PDF ePub
ePub Citation
Citation Print
Print


 XML Download
XML Download