Abstract
Purpose
Laparoscopic left lateral sectionectomy (LLLS) has been widely accepted due to benefits of minimally invasive surgery. Some surgeons prefer to isolate glissonian pedicles to segments II and III and to control individual pedicles with surgical clips, whereas opt like to control glissonian pedicles simultaneously using endoscopic stapling devices. The aim of this study was to find the rationale of LLLS using endoscopic staples.
Methods
We retrospectively analyzed and compared the clinical outcomes (operation time, drainage length, transfusion, hospital stay, and complication rate) of 35 patients that underwent LLLS between April 2004 and February 2012. Patients were dichotomized by surgical technique based on whether glissonian pedicles were isolated and controlled (the individual group, n = 21) or controlled using endoscopic staples at once (the batch group, n = 14).
Results
Mean operation time was 265.3 ± 21.3 minutes (mean ± standard deviation) in the individual group and 170 ± 22.9 minutes in the batch group. Operation time in the batch group was significantly shorter than the individual group (P = 0.007). Mean drainage length was 4.8 ± 1.6 and 2.6 ± 1.5 days in the individual and the batch group. There was significantly shorter in the batch group, also (P = 0.006). No transfusion was required in the batch group, but 4 patients in the individual group needed transfusion. Mean hospital stay was 10.7 ± 1.1 and 9.4 ± 0.8 days in the individual and the batch groups (P = 0.460). There were no significant complications or mortality in both groups.
Laparoscopic left lateral sectionectomy (LLLS) has been accepted with respect to its usefulness and benefits related to minimally invasive surgery [1,2,3]. After a consensus meeting held in 2008 (Louisiville statement), LLLS was introduced as a standard procedure for patients suitable for minimally invasive surgery [4]. Subsequently, Nguyen and Geller [5] also reported that LLLS is becoming a standard procedure and accounted for 20% of 3,000 laparoscopic liver resections performed until 2008. However, the technique required for this procedure continues to evolve and best practice has not been established.
Some surgeon prefer to use the Cavitron Ultrasonic Surgical Aspirator (CUSA, Salleylab, CO, USA) to isolate glissonian pedicles to segments II and III and control individual pedicles with surgical clips [6,7,8]. On the other hand, others prefer to control glissonian pedicles to segments II and III simultaneously using endoscopic stapling devices without isolating pedicles [9,10,11,12].
The aims of this study are to present our surgical outcomes for LLLS by surgical technique, that is, whether glissonian pedicles were isolated and controlled individually or whether endoscopic staples were used to control glissonian pedicles to segments II and III, and to provide a rationale for LLLS using endoscopic staples from the perspective of liver anatomy.
We retrospectively analyzed and compared clinical outcomes (operation times, drainage length, transfusion requirements, hospital stay, complication rate) in 35 patients that underwent LLLS between April 2004 and February 2012. Patients were dichotomized by surgical technique into an individual group (n = 21; glissonian pedicles isolated and controlled individually) or a batch (n = 14; glissonian pedicles simultaneously controlled using endoscopic staples). We included patients with a malignant tumor, intrahepatic lithiasis, and benign tumor within American Society of Anesthesiologists Classification 1, 2, but excluded those with portal vein (PV) thrombus, or an intrahepatic duct stone at the left main bile duct. All 35 patients were of Child-Pugh class A. There was no significant difference between the individual and the batch group (Table 1). Operation time, drainage length, hospital stay, transfusion during perioperative period, and postoperative complication were analyzed.
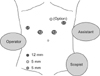
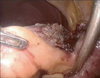
The procedures are both conducted with the patient supine in the reverse Trendelenburg position. LLLS is performed by two surgeons (operator, assistant) and one scopist. The operator stands to the right of the patient with the assistant and the scopist on the patient's left. Four ports are placed as shown in Fig. 1. Carbon dioxide pneumoperitoneum is maintained at 12 mmHg to minimize the risk of gas embolism. We use a 90' flexible scope and a 12-mm trochar is inserted in the supraumbilical area. An additional 12-mm trochar is inserted at around the intersection of the right subcostal and midclavicular lines and 5-mm trochar is inserted at around the intersection of the right subcostal and midaxillary lines. The assistant inserts the 5-mm trochar in the left subcostal area. The Pringle maneuver is not used during transaction of liver parenchyme. Intraoperative ultrasonography is performed to exclude any other lesion, to define tumor size, and to position and remark the resection anatomy. The falciform ligament and triangular ligament are divided with a Harmonic scalpel (Ethicon Endo-Surgery Inc., Cincinnati, OH, USA) up to the level of the inferior vena cava (IVC) and the left hepatic vein (LHV) through the right port. The left round ligament is divided up to the Arantius duct to free the left lateral segment and to allow easy stapling of the LHV. A Harmonic scalpel is used to dissect the anterosuperior portion of liver to reach umbilical plate (Fig. 2). After reaching the glissonian pedicle of left lobe (Fig. 3), glissonian pedicles to segments II and III are controlled using either of two methods.
In the individual group, glissonian pedicles were separately dissected and controlled and the LHV was controlled with Hemolock or metallic clips instead of the Endo-GIA. In the batch group, glissonian pedicles and surrounding liver tissue were controlled using an Endo-GIA (Ethicon Endo-Surgery Inc.) with a 60-mm white cartridge to the left side of the glissonian sheath (Fig. 4). In case of incomplete control of glissonian pedicles, we could perform the additional individual method or control with LHV simultaneously using endoscopic staples.
Remnant liver parenchyme was dissected with a Harmonic scapel, and then the LHV, within liver parenchyme, was divided using the Endo-GIA using a 60-mm white cartridge (Fig. 5).
Finally, in both group, specimen extraction was conduction using a protective bag through a supraumbilical port site subsequently extended by a midline incision. After careful hemostasis, an Argon beam was applied to the transection surface and fibrin glue was applied to staple lines. Prophylactic abdominal drains were placed in the transection surface (Fig. 6).
Statistical analysis was conducted using IBM SPSS Statistics ver. 19.0 (IBM Co., Armonk, NY, USA). Comparisons between nonparametric variables were analyzed using the Mann-Whitney U test and comparisons between categorical variables using the chi-square test. P-values of <0.05 were considered statistically significant.
Between April 2004 and February 2012, 35 patients (20 males, 15 females) underwent LLLS. Patient characteristics are summarized in Table 2.
In 21 patients, LLLS was performed for a malignant tumor, diagnoses were hepatocellular carcinoma (n = 10), metastasis from colorectal cancer (n = 8), cholangiocarcinoma (n = 1; diagnosed postopreatvely), biliary cystadenocarcinoma (n = 1), and combined hepatocellular carcinoma and cholangiocellular carcinoma (n = 1). In the other 14 patients, LLLS was performed for a benign lesion, diagnoses were intrahepatic lithiasis (n = 7), hemangioma (n = 3), a huge cyst (n = 3) and a pseudotumor (n = 1).
The surgical results are summarized in Table 3 and Fig. 7. The Individual method was conducted in 21 patients and the batch method was conducted in 14. Mean operation time was 265.3 ± 21.3 minutes (mean ± standard deviation) and 170.0 ± 22.9 minutes in the individual and the batch group and mean operation time was significantly shorter in the batch group (P = 0.007). Mean drainage length was 4.8 ± 1.6 and 2.6 ± 1.5 days in the individual and the batch group. There was significantly shorter in batch group, also (P = 0.006). Mean postoperative hospital stay was 10.7 ± 1.1 and 9.4 ± 0.8 days in the individual and the batch group. There was not significant differences between the individual and the batch group (P = 0.460). No transfusion was required in the batch group, but 4 of 21 patients in the individual group needed a blood transfusion during surgery. Postoperative laboratory findings between the individual and the batch group were not statistically significant. One open conversion due to tumor rupture occured in the individual group. Three cases of postoperative atelectasis were encountered in the individual group but only one in the batch group. We did not experience gas embolism, bile leakage, postoperative bleeding, bile duct injury, or vascular injury in either group. There was no postoperative death (defined as death during first 90 postoperative days). The average number of the Endo-GIA we used in the batch group was 2.92 ± 0.14.
In terms of medical cost, the batch group tends to be more expensive compared to the individual group [9]. However, since medical insurance system in South Korea reduces the burden of medical cost in patients, we believe the medical cost needs not to be considered otherwise at least in our country.
Since the first report of LLLS in 1996 [13], LLLS has become a popular treatment option, and many authors have reported on its feasibility and safety [1,2,3,9,11,12]. Currently, LLLS is accepted as a standard anatomic liver resection technique for any lesions located in segments II and III [2,10].
LLLS is a suitable procedure for training of laparoscopic hepatectomy, because it contains various procedures such as liver mobilization, liver parenchyme transection, and control of glissonian pedicles and vessels. We used the individual method to gain surgical experience in the early period, and later, that we randomly divided patients by surgical techniques (the individual group and the batch group) depending on surgeon's preference during the surgery. For this reason, the individual group is larger than the batch group, and patients in the upcoming future will be treated, preferably, with batch method.
Using the endoscopic stapling device to control all glissonian pedicles simultaneously, we could fasten the operation, but we had been worry about bile leakage, bleeding, or injury of glissonian pedicles to segment IV. Although the small number of cases treated (n = 14) preclude conclusions, we did not encounter any significant morbidity, such as, bleeding, bile leakage, or injury of glissonian pedicles to segment IV. Accordingly before concluding that LLLS using stapling devices is a straightforward, reproducible technique, we reviewed the published literatures to provide a rationale for our technique from an anatomic perspective.
Cho et al. [14,15] introduced three types of left biliary and portal systems and reported that regardless of type, segment IV ducts joined medially to the umbilical portion. Gumbs et al. [3] and Linden et al. [11] also supported our rationale for endoscopic stapling technique. During LLLS, the surgical resection margin is located at the left side of the umbilical portion, injury of segment IV duct is not an issue. Furthermore, variations of the PV are less than that those of bile duct, and the branch of the left PV to segment IV originates at the right side of the umbilical portion, and thus, injury of the PV is also unlikely. The arterial anatomy presented several variants that required modification of the dissection. In patients with a normal anatomy, there is a single branch to segment IV. In patients with a left hepatic artery originating from the left gastric artery, the artery to segment IV can always be preserved, and such patients, the arterial branch to segment IV originates at the right side of the umbilical portion, and thus, there is no need to consider injury of the arterial branch to segment IV [16]. Although it has a little risk, the stapler technique can result in injury of the arterial branch to segment IV, and result in atropy of segment IV. However, it does not cause any significant morbidity [17].
In this study, we report that portal pedicles, the LHV can be dissected using endoscopic staplers without bleeding or bile leakage. The LHV is the most critical structure and failure to secure it can lead to massive bleeding and gas embolism. However, dissection of the left round ligament and triangular ligament up to the level of the Arantius duct and the IVC enables staples to be easily placed through the LHV and ensure security. Furthermore, laparoscopic ultrasound examination, allow more accurate evaluation of the LHV and increases procedural safety.
For the entire batch group, we used Endo-GIA with a 60-mm white cartridge. There were no significant postoperative complications, including bile leakage or bleeding etc. A white cartridge has a 2.5-mm open staple height and a 1.0-mm closed staple height. We think that in patients with thicker pedicle, blue or gold cartridge can be used to prevent bile leakage due to duct disruption or delayed opening, but we are worry about bleeding from main pedicle.
Left PV thrombosis can be happened after the staple procedure in LLLS, especially, when the staple is very close to umbilical portion or injury the right side of pedicles. The distance from umbilical portion has not been measured in our study, just we did were that (1) tried to start liver parenchymal dissection a little bit left side of falciform ligament and (2) tried to apply staple a little bit left side of umbilical portion, not to involve the middle portion. Anyway at this moment, it is difficult to define what distance from umbilical portion is acceptable to prevent PV thrombosis and further research should be conducted in the upcoming future.
Recently we changed our surgical treatment strategy for intrahepatic stone. For the patients with stricture at the main junction of segments II or III glissonian pedicle, we tried laparoscopic left hemihepatectomy of liver because of hidden malignancy. In this study, 3 cases of intrahepatic stone were enrolled in the batch group. Fortunately our cases did not have any serious complication. But we are ought to remember risk of hidden malignancy, left hemihepatectomy rather than LLLS can be performed in patients with intrahepatic stone depending on the surgeon's experience and decision.
In patients with chronic hepatitis or cirrhosis, individual method often causes excessive blood loss and ascites accumulation because of pre-existing coagulopathy and aggravation of portal hypertension [18].
Mean operation time and mean drainage length in the batch group were significantly shorter than in the individual group (170.0 ± 22.9 and 265.3 ± 21.3 minutes, P = 0.007; 2.6 ± 1.5 and 4.8 ± 1.6 days, P = 0.006). Mean hospital stay was similar in the batch and the individual group (9.4 ± 0.8 and 10.7 ± 1.1 days, P = 0.460). Mean hospital stay was somewhat long, which we attribute to regional circumstances and customs. No significant intergroup difference was found for transfusion or postoperative laboratory finding and complications. We did not encounter conversion cases (batch to individual or individual to batch technique). Even though our cases were not large enough to conclude, depending on our experience, LLLS with batch method was done without conversion or interruption. These can lead to gain confidence with our surgical technique.
LLLS can be regarded as anatomic resection of liver, we believe that it's surgical result for malignant tumor is at least compatible to open surgery [19]. But our cases were not enough and we did not analyze the surgical results of open left lateral sectionectomy of liver during the same study period. Because most of cases at that period were done with laparoscopic technique. Furthermore, main focus of this study is surgical technique for LLLS.
In conclusion, our experience indicates that LLLS using endoscopic staples can be performed without extensive vascular isolation, and with acceptable low morbidity. Furthermore, this technique was found to be easier and safer technique and to have better outcomes without morbidity or mortality. In our opinion, LLLS using endoscopic staples should be considered a standard procedure for LLLS.
Figures and Tables
Fig. 2
Dissection of liver parenchyme using a Harmonic scalpel (Ethicon Endo-Surgery Inc., Cincinnati, OH, USA). Starting just left of the falciform ligament.
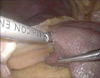
Fig. 4
(A) Transection of glissonian pedicles to segments II and III using an Endo-GIA (Ethicon Endo-Surgery Inc., Cincinnati, OH, USA) and a 60-mm white cartridge. (B) Transection of glissonian pedicles to segments II and III using an Endo-GIA and a 60-mm white cartridge. Arrows: staple lines after using an Endo-GIA.
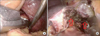
Fig. 5
Transection of the left hepatic vein using an Endo-GIA (Ethicon Endo-Surgery Inc., Cincinnati, OH, USA) and a 60-mm white cartridge.
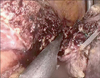
Fig. 6
Postoperative finding of laparoscopic left lateral sectionectomy using an Endo-GIA (Ethicon Endo-Surgery Inc., Cincinnati, OH, USA) and a 60-mm white cartridge. Arrow: The staple line is always located at the lowermost portion of transected liver parenchyma.
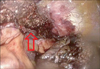
Fig. 7
Postoperative laboratory finding in the individual group and batch group. There was no significant difference between the individual and the batch group (A, P = 0.584; B, P = 0.617; C, P = 0.107). POD, postoperative day.

References
1. Abu Hilal M, Pearce NW. Laparoscopic left lateral liver sectionectomy: a safe, efficient, reproducible technique. Dig Surg. 2008; 25:305–308.
2. Chang S, Laurent A, Tayar C, Karoui M, Cherqui D. Laparoscopy as a routine approach for left lateral sectionectomy. Br J Surg. 2007; 94:58–63.
3. Gumbs AA, Gayet B, Gagner M. Laparoscopic liver resection: when to use the laparoscopic stapler device. HPB (Oxford). 2008; 10:296–303.
4. Buell JF, Cherqui D, Geller DA, O'Rourke N, Iannitti D, Dagher I, et al. The international position on laparoscopic liver surgery: The Louisville Statement, 2008. Ann Surg. 2009; 250:825–830.
5. Nguyen KT, Geller DA. Laparoscopic liver resection: current update. Surg Clin North Am. 2010; 90:749–760.
6. Belli G, Gayet B, Han HS, Wakabayashi G, Kim KH, Cannon R, et al. Laparoscopic left hemihepatectomy a consideration for acceptance as standard of care. Surg Endosc. 2013; 27:2721–2726.
7. Yamamoto M, Katagiri S, Ariizumi S, Kotera Y, Takahashi Y. Glissonean pedicle transection method for liver surgery (with video). J Hepatobiliary Pancreat Sci. 2012; 19:3–8.
8. Lesurtel M, Cherqui D, Laurent A, Tayar C, Fagniez PL. Laparoscopic versus open left lateral hepatic lobectomy: a case-control study. J Am Coll Surg. 2003; 196:236–242.
9. Wang X, Li J, Wang H, Luo Y, Ji W, Duan W, et al. Validation of the laparoscopically stapled approach as a standard technique for left lateral segment liver resection. World J Surg. 2013; 37:806–811.
10. Hasegawa Y, Nitta H, Sasaki A, Takahara T, Ito N, Fujita T, et al. Laparoscopic left lateral sectionectomy as a training procedure for surgeons learning laparoscopic hepatectomy. J Hepatobiliary Pancreat Sci. 2013; 20:525–530.
11. Linden BC, Humar A, Sielaff TD. Laparoscopic stapled left lateral segment liver resection: technique and results. J Gastrointest Surg. 2003; 7:777–782.
12. Khan AZ, Prasad KR, Lodge JP, Toogood GJ. Laparoscopic left lateral sectionectomy: surgical technique and our results from Leeds. J Laparoendosc Adv Surg Tech A. 2009; 19:29–32.
13. Azagra JS, Goergen M, Gilbart E, Jacobs D. Laparoscopic anatomical (hepatic) left lateral segmentectomy-technical aspects. Surg Endosc. 1996; 10:758–761.
14. Cho A, Okazumi S, Yoshinaga Y, Ishikawa Y, Ryu M, Ochiai T. Relationship between left biliary duct system and left portal vein: evaluation with three-dimensional portocholangiography. Radiology. 2003; 228:246–250.
15. Cho A, Okazumi S, Miyazawa Y, Makino H, Miura F, Ohira G, et al. Proposal for a reclassification of liver based anatomy on portal ramifications. Am J Surg. 2005; 189:195–199.
16. Broelsch CE, Whitington PF, Emond JC, Heffron TG, Thistlethwaite JR, Stevens L, et al. Liver transplantation in children from living related donors. Surgical techniques and results. Ann Surg. 1991; 214:428–437.
17. Shirouzu Y, Ohya Y, Hayashida S, Yoshii T, Asonuma K, Inomata Y. Reduction of left-lateral segment from living donors for liver transplantation in infants weighing less than 7 kg: technical aspects and outcome. Pediatr Transplant. 2010; 14:709–714.
18. Kaneko H, Otsuka Y, Takagi S, Tsuchiya M, Tamura A, Shiba T. Hepatic resection using stapling devices. Am J Surg. 2004; 187:280–284.
19. Kim SJ, Jung HK, Lee DS, Yun SS, Kim HJ. The comparison of oncologic and clinical outcomes of laparoscopic liver resection for hepatocellular carcinoma. Ann Surg Treat Res. 2014; 86:61–67.




 PDF
PDF ePub
ePub Citation
Citation Print
Print





 XML Download
XML Download