Abstract
Purpose
The aim of this study was to analyze the clinicopathological characteristics of solid pseudopapillary tumor (SPT) of the pancreas and to utilize an immunohistochemical panel to identify specific markers of the disease.
Methods
Eleven patients diagnosed with and treated for SPT of the pancreas over the past 15 years were retrospectively analyzed.
Results
The 11 patients consisted of 8 females and 3 males, of mean age at operation of 13.5 years (range, 10 to 18 years). The most frequent presenting symptom was abdominal pain and/or mass. One patient was referred with hemoperitoneum due to traumatic tumor rupture. The lesions were located in the body, head and tail of the pancreas in four, four, and three patients, respectively. Mean tumor diameter was 7.9 cm (range, 2.5 to 15 cm). Surgical procedures included distal pancreatectomy with splenectomy in four patients, pylorus preserving pancreaticoduodenectomy in four, distal pancreatectomy in two, and subtotal pancreatectomy with splenectomy in one. Mean follow-up was 60.5 months (range, 15 to 126 months). All patients remain alive without tumor recurrence. Immunohistochemical staining showed that all tumors were positive for β-catenin, progesterone receptor (PR), vimentin, and CD99. However, all tumors were negative for E-cadherin and cytokeratin 7 expression.
Conclusion
Patients with SPT of the pancreas have an excellent prognosis after surgical excision. Immunohistochemically, E-cadherin/β-catenin, PR, vimentin, and CD99 would help establish the diagnosis of SPT of the pancreas, although the results of immunohistochemical staining were found to have an indistinct complex immunoprofile.
Solid pseudopapillary tumor (SPT) of the pancreas is a rare clinical entity, with a reported incidence of 2%-3% of all pancreatic tumors [1-6]. SPT of the pancreas is regarded as having low-grade malignant potential, with most patients showing good long-term survival after surgical resection [7]. Although SPT of the pancreas has a favorable prognosis, 19.5% of affected patients have metastases or invasion to the liver, portal vein, and other organs [7].
The number of studies of SPT of the pancreas has increased markedly over the last 10 years, due to greater awareness and greater availability of immunohistochemical stains. It is imperative to differentiate SPT of the pancreas from other pancreatic tumors which demonstrate a different biologic behavior. Immunohistochemical study can provide valuable information to help in distinguishing SPT of the pancreas from other pancreatic tumors. Several antibodies have been utilized in the immunohistochemical evaluation of SPT of the pancreas, but no single marker has yet been shown specific to these tumors [8]. In this study, to further delineate the natural history of SPT of the pancreas and to determine a specific panel of immunohistochemical markers to identify the disease, we describes the clinicopathologic features and immunohistochemical expression profiles of 11 pediatric patients with SPT of the pancreas treated at our institution.
Eleven patients with a definitive pathological diagnosis of SPT of the pancreas who underwent surgical treatment at Kyungpook National University Medical Center during the 15-year period from 1995 to 2010 were included. The study was approved by the Ethics Committee of Kyungpook National University Medical Center. Patient demographics, clinical presentation, tumor localization and size, diagnostic methods, surgical treatment, and follow-up were reviewed from hospital records. SPT of the pancreas was diagnosed based on its gross and microscopic appearance as well as on immunohistochemical staining. Tumors were assumed to be malignant if there was extrapancreatic or pancreatic parenchymal invasion, lymph node involvement, perineural or vascular invasion, or distant metastases.
Immunohistochemical assays were performed using an automated Benchmark platform (Ventana Medical Systems, Tucson, AZ, USA) according to the manufacturer's recommendations. Four-micrometer-thick sections were immunostained with antibodies to chromogranin-A, synaptophysin, progesterone receptor (PR), c-kit, β-catenin, cytokeratin (CK), CK7, CD10, E-cadherin, CD99 and vimentin using an UltraView universal DAB detection kit (Ventana Medical Systems). All immunohistochemically stained slides were evaluated by a single pathologist. For all antibodies, staining of more than 5% of the tumor cells was regarded as positive.
For PR, cells showing nuclear staining were considered positive. For β-catenin, cells showing cytoplasmic/nuclear staining were considered positive. Cells were considered positive for E-cadherin, CK, and CK7 when the cytoplasmic membrane was stained and were considered positive for c-kit when cytoplasmic staining with or without nuclear staining was observed. Tumor cells were considered positive for chromogranin-A and synaptophysin when cytoplasmic granular staining was observed and were considered positive for CD99 when a paranuclear dot-like pattern was present. Cells were regarded as positive for vimentin and CD10 when cytoplasmic staining was observed.
Table 1 summarizes the clinicopathological characteristics of the 11 patients with SPT of the pancreas. The 11 patients included eight females and three males, with a male-to-female ratio of 1:2.7. Their mean age at the time of operation was 13.5 years (range, 10 to 18 years). Seven patients (64%) presented with an abdominal pain and/or mass. Other symptoms included nausea and vomiting. In two patients, tumors were detected incidentally during routine work-up for fatty liver (patient 6) and an ovarian mass (patient 8). One patient (patient 4) was referred with hemoperitoneum due to traumatic tumor rupture. The lesions were located in the body, head, and tail of the pancreas in four, four, and three patients, respectively. Mean tumor diameter was 7.9 cm (range, 2.5 to 15 cm). Preoperative serum tumor markers were assessed in 10 of the 11 patients, including α-fetoprotein (AFP) in seven patients, carcinoembryonic antigen (CEA) in 9, carbohydrate antigen (CA) 19-9 in 10, and CA 125 in 5. AFP, CEA, and CA 125 concentrations were within normal ranges in all patients tested. However, CA 19-9 was elevated in 3 of 10 patients (patient 8, 97.5 U/mL; patient 9, 40.6 U/mL; patient 10, 83 U/mL; normal range, <37 U/mL).
Abdominal ultrasonography (USG) and computed tomography (CT) scan were utilized to detect the mass in all 11 patients. Three patients underwent additional magnetic resonance imaging (MRI) and one underwent positron emission tomography.
Only one patient (patient 6) underwent preoperative USG-guided fine needle aspiration cytology (FNAC), which confirmed the diagnosis of SPT of the pancreas.
Surgical procedures included distal pancreatectomy with splenectomy in four patients, pylorus preserving pancreaticoduodenectomy in four, distal pancreatectomy in two, and subtotal pancreatectomy with splenectomy in one. One patient (patient 4) who presented with hemoperitoneum due to tumor rupture underwent an initial exploratory laparotomy and bleeding control because the mass was unresectable, followed by postoperative 3 cycles of chemotherapy (ifosfamide, etoposide, cisplatinum). Following tumor shrinkage, as shown by a repeat abdominal CT scan, this patient underwent a second exploratory operation 3 months later, during which subtotal pancreatectomy with splenectomy was performed. During follow-up, portal vein thrombosis and liver metastasis were observed, for which this patient underwent radiofrequency ablation and chemotherapy. At present, 97 months later, this patient is disease free, although she has portal vein obliteration and cavernous transformation.
All patients showed pathological findings typical of SPT of the pancreas. The tumors were composed of uniform cells with ovoid nuclei and eosinophilic granules, arranged in sheets with pseudopapillary architecture (Fig. 1). One patient (patient 4) was diagnosed with malignant SPT of the pancreas due to portal vein thrombosis and liver metastasis.
There were no perioperative deaths. Postoperative complications occurred in two patients. Patient 4 experienced portal vein obliteration and cavernous transformation, whereas patient 10 had a pancreatic pseudocyst, which was treated by cystogastrostomy 5 months after the initial operation. There was no recurrence at a mean follow-up of 60.5 months (range, 15-126 months). All 11 patients remain alive with no evidence of tumor recurrence.
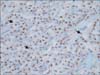
The detailed immunohistochemical results are listed in Table 2. Cytokeratin profiles (CK, CK7) characterize ducts and ductular cells of the pancreas. Chromogranin A and synaptophysin are considered neuroendocrine markers. Immunohistochemical analysis was performed on samples from all 11 patients. All were positive for vimentin, β-catenin, PR, and CD99. CD99 was stained as the intracytoplasmic paranuclear dot-like pattern (Fig. 2). Ten patients (91%) were positive for chromogranin A, 9 (82%) were positive for CK, 7 (64%) each were positive for c-kit and CD10, and 4 (36%) were positive for synaptophysin. All 11, however, were negative for E-cadherin and CK7 expression. All 11 displayed the characteristic cytoplasmic/nuclear immunoreactivity of β-catenin and loss of membranous E-cadherin.
SPT of the pancreas is a rare primary neoplasm of the pancreas, accounting for 2%-3% of all primary pancreatic neoplasms in all age groups [1-5]. Although up until a decade ago there were few studies of these tumors, the number of studies has increased markedly since that time due to improved awareness of these tumors. This has resulted from the standardization of the nomenclature and the greater availability of immunohistochemical stains [5-7].
Three patients previously misdiagnosed with nonfunctioning islet cell tumors were shown in 1959 to be a new entity, called Frantz's tumors [2]. These tumors have been given many names such as papillary cystic epithelial neoplasms, solid and papillary neoplasms, papillary cystic tumors, solid and cystic tumors, solitary cystic tumors, solid and papillary epithelial neoplasms, solid and cystic papillary epithelial neoplasms, solid papillary cystic tumors [2,3]. Recently, however, the World Health Organization recognized that all were SPTs of the pancreas [9].
Although SPT of the pancreas can affect patients at any age, most occur in young women in their second or third decade of life [10]. A large review reported that the mean age of patients with SPT of the pancreas is 21.97 years and that the male-to-female ratio is 1:9.78 [7]. Another review, of 292 patients with SPT of the pancreas, reported that mean patient age was 23.9 years, with a male-to-female ratio of 1:9.4 [11]. We observed a male-to female ratio of 1:2.7, similar to ratios reported in other pediatric case series, including 1:2 [12], 1:3.6 [13], 1:6.5 [14], 1:1.75 [15], and 1:4.5 [16]. Although each pediatric case series included only a small number of patients, taken together they show that female prevalence was lower in children than in adults.
The clinical presentation of SPT of the pancreas has been found to range from vague gastrointestinal symptoms such as upper abdominal fullness or discomfort to epigastric or left upper quadrant abdominal pain caused by an enlarged and often palpable abdominal mass [16]. A large literature review [7] reported that the most common clinical findings were abdominal pain (46.5%) and mass (34.84%), comparable with symptoms in our patients. However, presentations in adults and children have been reported to differ [14], with a palpable mass being the most common presenting symptom in children (60.0%) and an incidentally detected pancreatic mass the most common in adults (38.3%). Due to their slow growth, SPTs of the pancreas often remain asymptomatic until they have enlarged considerably and therefore may be detected incidentally during routine examination for unrelated diseases. Indeed, tumors in two of our patients were identified incidentally during abdominal USG or CT evaluation for fatty liver (patient 6) and an ovarian teratoma (patient 8). Occasionally, tumors may rupture due to a traumatic or unidentifiable cause, resulting in hemoperitoneum [7,10,11]. Indeed, one of our patients (patient 4) experienced hemoperitoneum secondary to traumatic tumor rupture.
Previous reviews [7,11] have found that 32%-34% of these tumors were located in the head of the pancreas, 26.2%-35.9% in the tail, 13.8%-14.8% in the body, 10.3%-24% in the body and tail, and 3.05%-4% in the head and body, findings was consistent with our result, in which 36.4% of the tumors were located in the head, 36.4% in the body, and 27.2% in the tail of the pancreas. In contrast, the most common frequent tumor location was reported to differ in children and adults, being the pancreatic head (66.7%) in children and the body or tail (80.9%) in adults [14]. While most of these tumors arise in the pancreas, others have been found in extrapancreatic locations, such as the retroperitoneum [17], mesocolon [18], and liver [19].
Preoperative serum tumor markers are usually within normal ranges [15,16,18]. Interestingly, we found that 3 of 10 patients (patients 8, 9, and 10) had elevated CA 19-9, although AFP, CEA and CA 125 concentrations were within normal ranges. Similarly, serum CEA concentration was elevated in 1 of 12 patients and serum CA 19-9 in 1 of 11 [20]
A variety of imaging modalities, including abdominal USG, CT, and MRI, have been used to characterize pancreatic masses preoperatively [21]. In addition, FNAC under radiological guidance, either USG or CT, has been used for preoperative diagnoses. Diagnostic cytological features include the presence of intracytoplasmic inclusions, multinucleated giant cells, and nuclear folds or grooves. Based on the preoperative FNAC, preoperative diagnosis of SPT of the pancreas was correctly made in 10 of 18 patients (55.6%) [22]. However, this procedure is invasive.
The exact cellular origin of SPT of the pancreas remains unclear [23]. Acinar, ductal, endocrine, and multipotential stem cells have been proposed as precursors of these tumors, with many attempts to correlate the immunoprofile of SPT of the pancreas with precursor cell types [3,8,15,21]. Immunohistochemically, SPTs of the pancreas have been found to variably express exocrine, endocrine, mesenchymal, and even epithelial cell markers. SPTs of the pancreas were found to have an indistinct complex immunoprofile, with 93% positive for neuron-specific enolase and 0% for chromogranin A [23], not consistent with pancreatic neuroendocrine cell types. Our patients' immunoprofiles were similar to those in previous reports [15,21-23], with only 36% and 91% of the tumors positive for the neuroendocrine markers synaptophysin and chromogranin A, respectively. Intracytoplasmic paranuclear dot-like CD99 expression was shown recently to differentiate SPT of the pancreas from other tumors [8], consistent with our results.
The pathogenesis of SPT of the pancreas is unclear. Wnt signaling associated with a β-catenin (CTNNB1) mutation, which leads to diffuse cytoplasmic and aberrant nuclear expression of β-catenin and a lack of membranous E-cadherin, plays a major role in the tumorigenesis of the SPT of the pancreas [24,25]. A hormonal influence in its pathogenesis has been suggested because of its female predominance.
Surgery is the mainstay of treatment. In contrast to pancreatic ductal carcinomas, large tumor size or vascular involvement, including involvement of the portal vein or superior mesenteric artery, does not preclude surgical resection [7]. Extensive lymphatic dissection and more radical local approaches are not indicated [7]. Moreover, in contrast to other pancreatic malignancies, surgical debulking should be performed for metastases. Therefore, the ideal treatment of SPT of the pancreas is complete resection of the tumor while preserving as much pancreatic tissue as possible. Although SPTs of the pancreas are generally benign and indolent tumors, local recurrence and metastases have been reported. Metastases usually occur in the liver, regional lymph nodes, mesentery, omentum, and peritoneum. Metastases in childhood are extremely rare [14]. In general, patient prognosis is excellent with high long-term survival rates, even in patients with local invasion and recurrence as well as metastases.
Several studies have attempted to delineate predictive features of malignant SPT of the pancreas. Venous invasion, high nuclear grade, and prominent necrobiotic nests characterized by aggregates of cells with pyknotic nuclei and eosinophilic cytoplasm have been reported to be useful indicators of malignancy [26]. In addition, tumor size >5 cm was found to be a significant predictor of malignant potential [27]. In contrast, preoperative features, including age, sex, tumor size, tumor location, elevated CEA levels, and elevated CA 19-9 levels, were not predictive of malignant SPT of the pancreas [14]. Malignant behavior such as recurrence or metastasis could not be completely excluded even in the absence of pathological features suggesting malignant potential, and aggressive tumor behavior was not predictive. Therefore, all patients with SPT of the pancreas should be monitored carefully, regardless of malignant potential [14].
The roles of adjuvant chemotherapy and radiotherapy in the treatment of SPT of the pancreas are currently unclear. However, very few case reports or case series have described success with chemotherapy and radiotherapy [28-30]. However, it is hard to determine whether long-term patient survival was due to chemotherapy or the natural disease progression of SPT of the pancreas.
In conclusion, SPT of the pancreas is a rare indolent tumor with low-grade malignant potential that usually affects young females. Radical surgical resection should be performed to enhance long-term survival, even in patients with metastases, invasion of adjacent organs, or lymph node involvement. Adjuvant chemotherapy or radiotherapy should be considered in patients with recurrent or unresectable tumors, although the efficacy of these methods has not yet been established. Immunohistochemically, cytoplasmic/nuclear immunoreactivity of β-catenin and loss of membranous E-cadherin, PR, vimentin, and CD99 would help establish the diagnosis of SPT of the pancreas, although the results of immunohistochemical staining were found to have an indistinct complex immunoprofile.
Figures and Tables
Fig. 1
High power view, showing that the tumor cells have uniform round nuclei with eosinophilic cytoplasm and central microvascular core forming a pseudopapillary pattern (H&E, ×200).
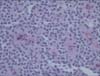
Fig. 2
CD99 immunohistochemical staining of solid pseudopapillary tumor of the pancreas. Tumor cells are stained in a paranuclear dot-like pattern (arrows) (×200).
References
1. Pettinato G, Di Vizio D, Manivel JC, Pambuccian SE, Somma P, Insabato L. Solid-pseudopapillary tumor of the pancreas: a neoplasm with distinct and highly characteristic cytological features. Diagn Cytopathol. 2002; 27:325–334.
2. Madan AK, Weldon CB, Long WP, Johnson D, Raafat A. Solid and papillary epithelial neoplasm of the pancreas. J Surg Oncol. 2004; 85:193–198.
3. Lam KY, Lo CY, Fan ST. Pancreatic solid-cystic-papillary tumor: clinicopathologic features in eight patients from Hong Kong and review of the literature. World J Surg. 1999; 23:1045–1050.
4. Chetty R, Serra S. Membrane loss and aberrant nuclear localization of E-cadherin are consistent features of solid pseudopapillary tumour of the pancreas: an immunohistochemical study using two antibodies recognizing different domains of the E-cadherin molecule. Histopathology. 2008; 52:325–330.
5. Morikawa T, Onogawa T, Maeda S, Takadate T, Shirasaki K, Yoshida H, et al. Solid pseudopapillary neoplasms of the pancreas: an 18-year experience at a single Japanese Institution. Surg Today. 2013; 43:26–32.
6. Patil TB, Shrikhande SV, Kanhere HA, Saoji RR, Ramadwar MR, Shukla PJ. Solid pseudopapillary neoplasm of the pancreas: a single institution experience of 14 cases. HPB (Oxford). 2006; 8:148–150.
7. Papavramidis T, Papavramidis S. Solid pseudopapillary tumors of the pancreas: review of 718 patients reported in English literature. J Am Coll Surg. 2005; 200:965–972.
8. Li L, Li J, Hao C, Zhang C, Mu K, Wang Y, et al. Immunohistochemical evaluation of solid pseudopapillary tumors of the pancreas: the expression pattern of CD99 is highly unique. Cancer Lett. 2011; 310:9–14.
9. Kloppel G, Solcia E, Longnecker DS, Capella C, Sobin LH. Histological typing of tumors of the exocrine pancreas (WHO institutional histological classification of tumors). 2nd ed. New York: Springer-Verlag;1996.
10. Soloni P, Cecchetto G, Dall'igna P, Carli M, Toffolutti T, Bisogno G. Management of unresectable solid papillary cystic tumor of the pancreas: a case report and literature review. J Pediatr Surg. 2010; 45:e1–e6.
11. Mao C, Guvendi M, Domenico DR, Kim K, Thomford NR, Howard JM. Papillary cystic and solid tumors of the pancreas: a pancreatic embryonic tumor? Studies of three cases and cumulative review of the world's literature. Surgery. 1995; 118:821–828.
12. Jung SE, Kim DY, Park KW, Lee SC, Jang JJ, Kim WK. Solid and papillary epithelial neoplasm of the pancreas in children. World J Surg. 1999; 23:233–236.
13. Choi SH, Kim SM, Oh JT, Park JY, Seo JM, Lee SK. Solid pseudopapillary tumor of the pancreas: a multicenter study of 23 pediatric cases. J Pediatr Surg. 2006; 41:1992–1995.
14. Lee SE, Jang JY, Hwang DW, Park KW, Kim SW. Clinical features and outcome of solid pseudopapillary neoplasm: differences between adults and children. Arch Surg. 2008; 143:1218–1221.
15. Speer AL, Barthel ER, Patel MM, Grikscheit TC. Solid pseudopapillary tumor of the pancreas: a single-institution 20-year series of pediatric patients. J Pediatr Surg. 2012; 47:1217–1222.
16. Zampieri N, Schiavo N, Capelli P, Scarpa A, Bassi C, Camoglio FS. Pseudopapillary tumor in pediatric age: clinical and surgical management. Pediatr Surg Int. 2011; 27:1271–1275.
17. Miyazaki Y, Miyajima A, Maeda T, Yuge K, Hasegawa M, Kosaka T, et al. Extrapancreatic solid pseudopapillary tumor: case report and review of the literature. Int J Clin Oncol. 2012; 17:165–168.
18. Ishikawa O, Ishiguro S, Ohhigashi H, Sasaki Y, Yasuda T, Imaoka S, et al. Solid and papillary neoplasm arising from an ectopic pancreas in the mesocolon. Am J Gastroenterol. 1990; 85:597–601.
19. Kim YI, Kim ST, Lee GK, Choi BI. Papillary cystic tumor of the liver: a case report with ultrastructural observation. Cancer. 1990; 65:2740–2746.
20. Goh BK, Tan YM, Cheow PC, Chung AY, Chow PK, Wong WK, et al. Solid pseudopapillary neoplasms of the pancreas: an updated experience. J Surg Oncol. 2007; 95:640–644.
21. Huang HL, Shih SC, Chang WH, Wang TE, Chen MJ, Chan YJ. Solid-pseudopapillary tumor of the pancreas: clinical experience and literature review. World J Gastroenterol. 2005; 11:1403–1409.
22. Butte JM, Brennan MF, Gonen M, Tang LH, D'Angelica MI, Fong Y, et al. Solid pseudopapillary tumors of the pancreas. Clinical features, surgical outcomes, and long-term survival in 45 consecutive patients from a single center. J Gastrointest Surg. 2011; 15:350–357.
23. Kosmahl M, Seada LS, Janig U, Harms D, Kloppel G. Solid-pseudopapillary tumor of the pancreas: its origin revisited. Virchows Arch. 2000; 436:473–480.
24. Huang SC, Ng KF, Yeh TS, Chang HC, Su CY, Chen TC. Clinicopathological analysis of β-catenin and Axin-1 in solid pseudopapillary neoplasms of the pancreas. Ann Surg Oncol. 2012; 19:Suppl 3. S438–S446.
25. Tanaka Y, Kato K, Notohara K, Hojo H, Ijiri R, Miyake T, et al. Frequent beta-catenin mutation and cytoplasmic/nuclear accumulation in pancreatic solid-pseudopapillary neoplasm. Cancer Res. 2001; 61:8401–8404.
26. Nishihara K, Nagoshi M, Tsuneyoshi M, Yamaguchi K, Hayashi I. Papillary cystic tumors of the pancreas. Assessment of their malignant potential. Cancer. 1993; 71:82–92.
27. Kang CM, Kim KS, Choi JS, Kim H, Lee WJ, Kim BR. Solid pseudopapillary tumor of the pancreas suggesting malignant potential. Pancreas. 2006; 32:276–280.
28. Fried P, Cooper J, Balthazar E, Fazzini E, Newall J. A role for radiotherapy in the treatment of solid and papillary neoplasms of the pancreas. Cancer. 1985; 56:2783–2785.
29. Strauss JF, Hirsch VJ, Rubey CN, Pollock M. Resection of a solid and papillary epithelial neoplasm of the pancreas following treatment with cis-platinum and 5-fluorouracil: a case report. Med Pediatr Oncol. 1993; 21:365–367.
30. Das G, Bhuyan C, Das BK, Sharma JD, Saikia BJ, Purkystha J. Spleen-preserving distal pancreatectomy following neoadjuvant chemotherapy for papillary solid and cystic neoplasm of pancreas. Indian J Gastroenterol. 2004; 23:188–189.




 PDF
PDF ePub
ePub Citation
Citation Print
Print




 XML Download
XML Download