Abstract
The lung, followed by regional lymph node and bone, is the most common site for extrahepatic metastasis of hepatocellular carcinoma (HCC). Metastatic skin lesion of HCC is rare, and it is a sign of poor prognosis, indicating the strong possibility of metastases in other regions of the body. We report the case of a 52-year-old male with multiple metastases, including skin metastasis of HCC, which were treated with multidisciplinary therapy.
According to the Barcelona Clinic Liver Cancer staging, extrahepatic metastasis of hepatocellular carcinoma (HCC) is a poor prognostic factor, and is regarded as advanced stage (C). Most patients with advanced stage receive palliative treatment. The most common site for extrahepatic metastasis of HCC is the lung, followed by regional lymph node, and bone [1,2]. Metastatic skin lesion of HCC is rare, accounting for less than 0.8% of all metastatic cutaneous tumors and occurring in 2.7%-3.4% of all HCCs [3,4]. Skin metastasis is a sign of poor prognosis, indicating the strong possibility of metastases in other regions of the body, and points to a median survival time of less than five months [5,6]. We report on a case of multiple metastases, including skin metastasis, which were treated with multidisciplinary therapy.
A 52-year-old male patient with a history of type B viral hepatitis visited the hospital with abdominal distension; at that time, a huge mass (a mass measuring approximately 7 cm on segment 8, and a mass measuring 2.5 cm on segment 6) in the right hemiliver and hematomas around the liver were observed on abdominal computed tomography (CT) scan; the diagnosis was HCC rupture (Fig. 1). After transarterial chemoembolization (TACE), segment 8 segmentectomy and segment 6 wedge resection were performed (Fig. 2); hyperthermic intraperitoneal adriamycin irrigation was then performed for prevention of peritoneal seeding. According to the biopsy report, there was no viable tumor because of TACE. The patient was discharged with no specific complication and no evidence of recurrence was observed for seven years postoperative (During the seven years, ultrasonography or CT and tumor marker check was performed every 3 months, but there was no abnormal finding). However, 84 months after the initial operation, an abnormal splenic mass was observed on follow-up ultrasonography, and his tumor marker was elevated (α-fetoprotein, 1.94; protein induced by vitamin K absence or antagonist II, 309). Therefore, we performed positron emission tomography-CT, and multiple metastases were observed. The sites of metastases were brain, adrenal gland, spleen, kidney, skin (buttock), back muscle, and buttock muscle (Figs. 3, 4); however, there was no intrahepatic recurrence. Skin biopsy confirmed metastasis of HCC (Fig. 5). The patient then received radiation therapy for brain metastasis, and took oral medication with sorafenib (Nexavar, Bayer Health Care, Leverkusen, Germany). After one month, a new lesion was observed on his right thumb (Fig. 6). However, follow-up abdominal CT showed stable condition of the previously mentioned multiple metastases (mass of spleen, left adrenal gland, left kidney, back muscle, and buttock muscle), and the brain mass had shrunk. We performed en bloc resection of intra-abdominal metastatic masses (splenectomy, left adrenalectomy, left nephrectomy) and excision of the right thumb mass (Fig. 7; Edmonson-Steiner grade of these masses are grade 2, and histologic type is trabecular, and cell type is hepatic cell). Currently, 18 months after initial diagnosis of multiple metastases, he is still alive, and he takes an oral medication with sorafenib for target therapy of remnant tumor in back muscle, buttock, and brain, which are unresectable (Fig. 8).
Metastatic skin lesion of HCC is rare; fewer than 50 such cases have been reported in the literature with most of them diagnosed histologically by excision biopsy specimen [7]. Cutaneous metastases originating from HCC appear as rather rapidly growing nodules, usually on the scalp, chest, and shoulder. They appear as single or multiple, firm, painless, nonulcerative, reddish-blue nodules, measuring 1 to 2.5 cm in diameter [8]. Especially in cases of skin metastasis of HCC caused by systemic spread of disease, indicating the strong possibility of metastases in other regions of the body, the prognosis is dismal [5,6]. In addition, in this case, the patient had multiple metastatic lesions, including skin metastasis. Despite these poor prognostic factors, this patient seemed to have good tumor biology, for example, 100% tumor necrosis after initial TACE, recurrence after long period, good response to treatment after multiple site recurrence, and not highly elevated AFP. This favorable tumor biology may be an important factor of long-term survival in this patient.
In treatment of advanced HCC, to date, the only drug that has successfully made it into a phase III trial is sorafenib. Sorafenib is an oral multikinase inhibitor blocking tumor cell proliferation and angiogenesis by targeting the serine/threonine kinases Raf-1/B-Raf and the tyrosine kinases of vascular endothelial growth factor receptor-2/-3 and Platelet-derived growth factor receptors-b. Median survival and the time to radiologic progression were nearly three months longer for patients treated with sorafenib than for those who received placebo [9]. In this case, after diagnosis of multiple metastases, he was treated with sorafenib. Although we performed successful resection of intraabdominal metastatic masses, remnant tumors remain (brain, back muscle, and buttock muscle). Therefore, further survival of this patient is mainly dependent on sorafenib.
Figures and Tables
Fig. 1
Initial computed tomography image. A huge mass was observed in segment 8 (A), and an additional mass in segment 6 (B). Perihepatic leakage of contrast (C) was also seen, compatible with rupture of hepatocellular carcinoma.
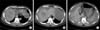
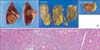
Fig. 2
(A) Gross findings after segmentectomy of segment 8, and wedge resection of segment 6. (B) Microscopic findings. Microscopic finding showed no viable hepatocellular carcinoma, believed to be due to initial transarterial chemoembolization (H&E: Left, ×10; Right, ×20).
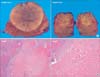
Fig. 3
Computed tomography image shows multiple extrahepatic metastases spleen (A), left adrenal gland (B), back muscle (C), buttock muscle (D), and skin of hepatocellular carcinoma (E).

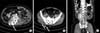
Fig. 4
Magnetic resonance imaging (A, focal strong enhancing mass at left precentral gyrus) and positron emission tomography image (B, focal fludeoxyglucose hot uptake nodule on left parietal lobe) of brain metastasis. Brain metastasis was treated with radiation therapy. After radiation therapy, tumor had shrunk (C)

Fig. 5
Skin lesion on computed tomography image (A, arrow) and excised mass on buttock (B). (C) Microscopic finding reveals skin metastasis of hepatocellular carcinoma (H&E: Left upper, ×10; Right upper, ×20; Left lower, ×20; Right lower, ×30).
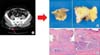
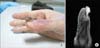
Fig. 6
(A) After diagnosis of multiple metastases, a new skin lesion was identified on the right thumb. (B) Magnetic resonance imaging also shows a pedunculated soft tissue mass.
References
1. Kanda M, Tateishi R, Yoshida H, Sato T, Masuzaki R, Ohki T, et al. Extrahepatic metastasis of hepatocellular carcinoma: incidence and risk factors. Liver Int. 2008; 28:1256–1263.
2. Uka K, Aikata H, Takaki S, Shirakawa H, Jeong SC, Yamashina K, et al. Clinical features and prognosis of patients with extrahepatic metastases from hepatocellular carcinoma. World J Gastroenterol. 2007; 13:414–420.
3. Brownstein MH, Helwig EB. Metastatic tumors of the skin. Cancer. 1972; 29:1298–1307.
4. Reingold IM, Smith BR. Cutaneous metastases from hepatomas. Arch Dermatol. 1978; 114:1045–1046.
5. Asselah T, Condat B, Cazals-Hatem D, Hassani Z, Bernuau J, Groussard O, et al. Ectopic hepatocellular carcinoma arising in the left chest wall: a long-term follow-up. Eur J Gastroenterol Hepatol. 2001; 13:873–875.
6. Okusaka T, Okada S, Ishii H, Nose H, Nagahama H, Nakasuka H, et al. Prognosis of hepatocellular carcinoma patients with extrahepatic metastases. Hepatogastroenterology. 1997; 44:251–257.
7. Magaña M, Gomez LM. Skin metastasis from hepatocarcinoma. Am J Dermatopathol. 2009; 31:502–505.
8. Reingold IM, Smith BR. Cutaneous metastases from hepatomas. Arch Dermatol. 1978; 114:1045–1046.
9. Galle PR. Sorafenib in advanced hepatocellular carcinoma: we have won a battle not the war. J Hepatol. 2008; 49:871–873.




 PDF
PDF ePub
ePub Citation
Citation Print
Print


 XML Download
XML Download