Abstract
Purpose
The purpose of this study is to analyze the treatment strategies of patients with endoscopic retrograde cholangiopancreatography (ERCP)-related perforations. This is a retrospective study.
Methods
We experienced 13 perforations associated with ERCP. We reviewed the medical recordsand classified ERCP-related perforations according to mechanism of injury in terms of perforating device. Injury by endoscopic tip or insertion tube was classified as type I, injury by cannulation catheter or sphincterotomy knife as type II, and injury by guidewire as type III.
Results
Of four type I injuries, one case was managed by conservative management after primary closure with a hemoclip during ERCP. The other three patients underwent surgical treatments such as primary closure orpancreatico-duodenectomy. Of five type II injuries, two patients underwent conservative management and the other three cases were managed by surgical treatment such as duodenojejunostomy, duodenal diverticulization and pancreatico-duodenectomy. Of four type III injuries, three patients were managed conservatively and the remaining patient was managed by T-tube choledochostomy.
Conclusion
Type I injuries require immediate surgical management after EPCP or immediate endoscopic closure during ERCP whenever possible. Type II injuries require surgical or conservative treatment according to intra- and retro-peritoneal dirty fluid collection findings following radiologic evaluation. Type III injuries almost always improve after conservative treatment with endoscopic nasobilliary drainage.
Endoscopic retrograde cholangiopancreatography (ERCP) is commonly performed to treat hepato-pancreato-biliary disease. The rate of ERCP-related bowel perforation is 0.3 to 1.0% [1-3]. The mortality rate in perforated patients is as high as 25% [4]. Many previous reports have described the management of perforation injuries associated with ERCP. Some have characterized treatment strategies according to location and mechanism of bowel perforation [5,6]. However, these classifications may be difficult to apply to real clinical situations because of their ambiguity, and therefore the most appropriate management strategy for ERCP-related perforations remains unclear. The purpose of this study is to analyze the treatment strategies and outcomes of patients with ERCP-related perforations based on a new classification.
Between April 1994 and December 2009, 7,638 cases of ERCP were performed. Among these patients, twelve patients (0.16%) experienced perforations that were associated with ERCP. One patient with suspected injury during ERCP was transferred to our hospital for management. The patient was included in our study. We retrospectively reviewed the medical records of 13 patients who were managed for perforations associated with ERCP.
We classified ERCP-related perforations according to mechanism of injury in terms of the perforating device. If bowel perforation was identified while the endoscope was inserted into the second portion of duodenum or while it was withdrawn from duodenum, the perforation was caused by the endoscopic blind tip or insertion tube. We classified this type of injury as type I.
If the injury was caused by a cannulation catheter or a knife for sphincterotomy, the injury was classified as type II.
Injuries caused by guidewires after cannulation of the papilla during exploration of the bile duct or pancreatic duct was classified as type III (Table 1, Fig. 1).
We analyzed data regarding the clinical manifestations, diagnostic methods, radiologic findings, methods of management, and clinical outcomes of all patients.
The sample included six male and seven female patients with a median age of 65.1 (±10.7) years. The objectives of ERCP were common bile duct stones (53.8%), jaundice with suspicious malignancy (38.5%) and cholangitis without stone (7.7%). Diagnoses of perforation were made during ERCP in six patients (46.2%). The other seven patients were diagnosed after ERCP by plain X-ray, abdominal computed tomography (CT) or sonography. Six of 13 patients (46.2%) were managed conservatively, while the other seven patients (53.8%) were managed by surgical treatment. There was no mortality (Table 2).
All four patients with type I injury were diagnosed with bowel perforation during ERCP procedure. One case improved with conservative management, while the other three cases were managed surgically. Of five type II injuries, three cases were detected immediately and the other two cases were detected late. Two cases improved with conservative management, while the other three cases were managed surgically. Of four type III injuries, most were detected immediately. Three cases improved with conservative management, while the last was managed surgically (Table 3).
Of four patients, only one was managed conservatively with endoscopic treatment. Endoscopic clipping was performed just after bowel perforation during ERCP. This patient improved without antibiotics or any drainage procedures.
Three patients underwent surgical treatment. Two patients underwent immediate surgery within threehours after ERCP. The operations were performed to achieve primary closure of the perforation site. One patient (patient No. 2), who was thought to have Klatskin tumor of Bisthmus type IIIbin pre-operative radiologic finding, received percutaneous transhepatic biliary drainage after primary closure of the perforation site. None of the patients who underwent immediate surgery experienced any complications or had problematic outcomes. One patient underwent a delayed operation because he had stable vital signs and the possibility of ampulla of Vater cancer requiring extensive operation. We considered elective Whipple's operation for this patient. In the operative findings, the tissue around the lesion was fragile and severely inflamed. Post-operative complications such as pancreatico-jejunostomy site leakage and wound dehiscence were observed. Biopsy reported duodenal wall defect with periduodenal abscess, epithelial hyperplasia in common bile duct and no tumor. Fortunately, the patient recovered well after wound closure under general anesthesia and conservative treatment for anastomosis site leakage.
Of five patients, two were managed conservatively. The cannulation of the ampulla of Vater failed in these patients. After ERCP, follow-up CT and simple X-ray showed retroperitoneal air and perirenal free air, but no fluid collection. Neither patient had leukocytosis. One patient received Levin tube drainage and the other was managed without any drainage procedures. Neither patient experienced any complications during hospital stay. One case was transferred to another hospital at the request of the patient.
Of five patients, three were managed by surgical treatment. One case underwent an immediate operation. Two cases were detected later and underwent delayed operations. In the immediately operated case, the perforation occurred because the cannula punctured the anterio-medial wall of the duodenum instead of the ampulla of Vater. The patient complained of abdominal pain immediately after ERCP and free air was detected on simple X-ray. After a little dissection of pancreatico-duodenal junction, we found an approximately 5 mm-sized perforation site. Duodeno-jejunostomy through perforation site, T-tube choledochostomy and cholecystectomy were performed, and the patient's condition improved after the operation. The reported biopsy was adenocarcinoma in fundus of gallbladder, invasion into perimuscular connective tissue and no tumor in resection margin of cystic duct. Another case was detected within a day after ERCP. Fluid collection in Morison pouch was detected inultrasonography and greatly increased in follow-up sonography two days after ERCP. The fluid looked like complicated ascites. Surgery was performed andwe detected retroperitoneal bile staining and saponification, but were unable to locate the bowel perforation. We believe that pancreatic and bile duct injury may have occurred as the cannula passed through the ampulla of Vater. Although we carried out multiple drainage procedures, the patient's condition worsened due to the development of a pancreatic fistula. We re-operated on postoperative day 11 and performed a duodenal diverticulization. The patient improved after the second operation. The last patient's injury was detected three days after ERCP. A common bile duct stricture was seen in the CT scan before ERCP. ERCP was performed with some difficulty. The endoscopist did not recognize the perforation during the procedure. Three days from ERCP, a CT was performed due to severe abdominal pain. The CT showed retroperitoneal air and fluid collection. Because the vital signs and symptoms of the patient were tolerable and common bile duct cancer was suspected, we decided to perform delayed extensive operation after conservative management. This operation was performed 10 days after ERCP. The perforation site was not identified, severe retroperitoneal inflammation remained and the duodenal wall was severely edematous. We carried out a pylorus-preserving pancreatico-duodenectomy. Biopsy reported strictureand chronic active inflammation with epithelial cell hyperplasia in common bile ductand chronic active inflammation with extensive abscess formation and serositis in duodenum. There was no tumor. The patient was discharged at post-operative day 37 without any complications.
Of four patients, three were successfully managed conservatively with endoscopic nasobilliary drainage (ENBD). Perforations by guidewire were identified by contrast extra-vasation during ERCP, unusual gas on CT and perirenal free air on simple X-ray. All three cases experienced no difficulties during the ERCP procedure.
One underwent surgical treatment. The patient experienced severe abdominal pain after ERCP. CT was performed immediately after ERCP and revealed massive pneumoperitoneum. The endoscopist had not experienced any difficulty during ERCP, and therefore did not recognize the perforation. We performed an immediate diagnostic laparotomy, but were unable to determine the exact location of the perforation. There was no fluid collection and no inflammation. We suspected bile duct injury, and performed a T-tube choledochostomy. After surgery, the patient was discharged without problems or any complications.
ERCP-related bowel perforations are very rare and unpredictable. There have been some reports about possible predisposing factors. Enns et al. [2] reported that factors associated with increased risk of ERCP-related bowel perforation included suspected sphincter of Oddi dysfunction, older age, a dilated bile duct, sphincterotomy, and longer duration of the procedure. Kayhan et al. [7] reported that the presence of duodenal anatomic abnormalities and peripapillary diverticulum were associated with complication. In the present study, among three patients with duodenal diverticulae, one patient (No. 4) experienced a duodenal diverticular perforation by endoscopic tip. Another patient (No. 1) had duodenal stricture due to a previous duodenal ulcer and experienced a duodenal perforation during endoscopic approach. Another patient (No. 3) underwent subtotal Billroth II gastrectomy due to gastric cancer and had an anatomic variation of the afferent jejunal loop. This afferent jejunal loop was torn by endoscopy during insertion. Another patient (No. 6) had an obscure ampulla. The cannula entered the ampulla only after several attempts. We believe that the injury around the ampulla was caused by the cannulation catheter during this procedure. Another patient (No. 9) experienced a cannula puncture of the ampulla of Vater but the guidewire did not enter the bile duct through the cannula. The endoscopist was unable to advance the endoscope to continue the procedure. We suspected that the retroperitoneal perforation around the ampulla was caused by the cannulation catheter.
Two previous studies introduced classifications of ERCP-related perforations based on anatomical location and mechanism of injury. Stapfer et al. [5] defined type I (lateral or medial wall perforation of duodenum), type II (peri-Vaterian injury), type III (distal bile duct injury) and type IV (retroperitoneal air alone) injuries. Howard et al. [6] classified type I (guidewire perforation), type II (periampullary retroperitoneal perforation) and type III (duodenal perforation remote from the papilla) injuries. We found Stapfer et al.'s classification to be ambiguous regarding the boundary between the anterior or posterior duodenum and the peri-Vaterian area. And Howard et al.'s classification was limited because the definition of 'remote' in type III injuries was not clear. Stafer's type I and Howard's type III also mentioned perforation within the duodenum. In our experience, one patient (No. 3) had anatomic variation due to a previous operation. It was difficulty to classify this patient according to Stapfer et al.'s or Howard et al.'s classification. This patient had a perforation not in duodenum but in jejunum of afferent loop. Previous reports focused on perforated location but we focusedon perforation size. The perforation size varied according to the device causing perforation. We simplified the classification of ERCP-related bowel perforations by basing our classification only on the mechanism of injury (Table 1, Fig. 1).
ERCP course could be divided to 3 steps. The 1st step is approaching the second portion of the duodenum. The 2nd step is cannulation of ampulla of Vater by catheter. Sphincterotomy could be done using sphincterotomy knife. The 3rd step is investigation of bile duct and pancreatic duct. The main device used differs from step to step. Type I injuries are induced by endoscopic tip or insertion tube. The diameter of endoscopic tip for ERCP is approximately equal to that of a finger, and the camera view is from the side unlike the usual gastroduodenoscopic tip view [8]. ERCP endoscopes are so thick and stiff that bowel injuries may be aggravated in proportion to the size of perforation. During advancement of the endoscope, the side of the bowel could be torn by insertion tube. Large perforations are not expected to heal without surgery due to severe intra-peritoneal contamination and sepsis. Therefore, we propose that exploratory laparotomy is a better choice for treatment of Type I injuries. In the present study, patients with type I injuries were all treated surgically except for one (No. 1). When that patient suffered an endoscopic injury on the duodenal wall, immediate endoscopic clipping was performed to limit intra-peritoneal contamination. The patient improved with conservative management. Siebert reported the successful use of an endoscopic clipping device to treat a duodenal perforation that occurred during an endoscopic ultrasound examination [9]. Mutignani et al. [10] described a duodenal perforation that occurred during ERCP that was sealed with fibrin glue and managed conservatively. If immediate closure by endoscopic methods is possible, conservative management without surgery may be the best treatment method. Of course, some cases require surgery after endoscopic clipping due to hemodynamic instability [8]. It is necessary to closely observe patients' vital signs after endoscopic closure of bowel perforations. Ryozawa et al. [11] reported that the development of double-balloon endoscopes had resulted in improved success rates for ERCP in patients with Roux-en-Y reconstruction. In our patient sample, one patient experienced duodenal perforation with Billroth II anasotmosis. We believe that it is advisable to use double-balloon endoscopy in cases with Billroth II anatomic variations.
Type II injuries are induced by sphincterotomy knives or cannulation catheters. Generally, the diameter of ERCP cannulation catheters is 5 to 7 Fr. This diameter is so small that perforations by cannulae may seal naturally. However, if significant bile or pancreatic juice leakage occurs, the healing of injured tissue due to irritant fluid would be difficult and emergency surgery should be considered. We believe that fluid collection in the intra- or retro-peritoneal cavity is a significant operative indication of type II injures induced by sphincterotomy knives or cannulation catheters. Stapfer et al. [5] reported that fluid collection in the retroperitoneal or peritoneal cavity is an indicator for surgery after ERCP-related duodenal perforation. Morgan et al. [8] reported that persistent collection of infected fluids collection can prevent the healing of the perforation site. Husain et al. [12] reported that 33% (7/21) of patients showed extra-luminal retroperitoneal air following endoscopic sphincterotomy and that this observation was not clinically significant. Stapfer et al. [5] insisted that retroperitoneal air alone probably requires no additional treatment or further work-up, if the findings of abdominal examinations are normal and there is no evidence or suspicion of contrast extravasation. In two of our patients (No. 5 and No. 6), CT findings showed retroperitoneal air after ERCP. But the patients' symptoms were mild. Vital signs and laboratory values were also normal. Although retroperitoneal air was observed in CT, there were no fluid collections in retroperitoneal or intra-abdominal cavity. We tried conservative managementand these treatments were done successfully. In type II injuries when the patient is stable and has no fluid collection and only retroperitoneal air, conservative management may be possible. In the case of patient No.7, she showed not retroperitoneal air but intra-peritoneal air. Because of suspected panperitonitis on physical examination, we decided on emergency operation without follow-up CT scan. We found bile leakage in the pancreatico-duodenal junction. If the patient had CT evaluation, fluid collection would have shown because bile leakage was observed at that time. We dissected the pancreatico-duodenal junction while towing the duodenum. After a minor dissection, we could find an approximately 5 mm sized perforation site in the anterio-medial portion of the duodenum. The perforation was caused by cannulation catheter according to the endoscopist and the diameter of catheter was 1.8 mm. We thought that the perforation size could have been enlarged to 5 mm due to the procedures of lateral traction and dissection. We performed duodenojejunostomy for duodenal perforation. Some surgeons recommend primary closure and drainage in the case of early detection [13]. However, we prefer duodenojejunostomy. This procedure is thought to have the benefit of decompression of duodenal pressure through side-to-side anastomosis. The procedure is not so difficult and does not take very long; about 15 minutes. Duodenojejunostomy could be another method in duodenal injury that requires operation.
Type III injuries are induced by guidewires after cannulation of the ampulla. The diameter of the guidewire is smaller than that of a cannulation catheter, and therefore perforations may be small and the location of perforation might be in the common bile duct or pancreatic duct passing through the ampulla of Vater. If ENBD was maintained, the pressure and flow in injured ducts might be lower than in injured ducts without ENBD. The possibility of being sealed-off is high and inflammation may be mild. Therefore, the success rate of conservative management may be higher than in other types of bowel perforation. Howard et al. [6] reported that patients who suffered guidewire perforations resolved with conservative treatment. In our study, all cases, except one (No. 13), were treated by conservative management with ENBD. This exceptional case had immediate operation due to severe abdominal pain and intra-abdominal free air. We presumed that the perforation was caused by the guidewire, because insertion of endoscopy to the duodenum was smooth and the cannulation of the ampulla was uneventful. In operation, we observed little inflammation around the operative field and were unable to find the location of the bowel perforation. The patient improved after T-tube choledochostomy. We thought that the effect of this operation would be similar to ENBD to decrease flow and pressure of common bile duct. Chung et al. [14] reported that improvement of symptoms within 24 hours was correlated with spontaneous recovery. Neither the presence of retroperitoneal air nor contrast leaks was predictive of the need for surgery. In our cases, we did not observe retroperitoneal air, but observed intra-abdominal free air. It was difficult to decide whether the immediate exploratory laparotomy of our patient was truly necessary. We think that conservative management with ENBD might have been sufficient after 24 hours observation in patient number 13. When intra-abdominal free air occurs due to injury by guidewire, it is thought that conservative management with ENBD is possible.
One patient (No. 4) in type I injuries and two patients (No. 8 and No. 9) in type II injuries underwent delayed operations. We performed a delayed operation due to the preparation required for extensive surgery on suspicious malignancies. We were immediately unable to operate on the patients with suspicious malignancy because we considered the preparations for anesthesia and operation team of one-stage operations of malignancy to be insufficient. But the results of delayed operation were unsatisfactory. These patients had severe inflammation in spite of conservative management and difficulties were encountered during surgery due to fragile tissues and adhesion. And post operative complications like leakage and fistula occurred in delayed operation. In contrast, the patients who received an immediate operation (No. 2, 3, 7) experienced only mild inflammation and had relatively fresh tissue, so surgeries were uneventful and post-operative recoveries were satisfactory. Avgerinos et al. [15] insisted that the interval between perforation and operation was of great significance. Mortality rates increase dramatically with delayed surgical management (>24 hours). During conservative management, patients' vital signs remained stable and pains were alleviated. We thought that the reason for the resolved pain and stable vital signs during conservative management would be not intra-peritoneal perforation but, retroperitoneal perforation. Because the retroperitoneal area is a trapped space, the abscess is likely to be localized. When the operation was done in the 3 or 10 days of conservative management, it was difficult to deal with remaining inflammatory tissues. We could not perform the previously planned extensive surgery in patient-number 8 because of severe tissue inflammation. Considering the operative findings in patients with delayed operation (No. 4, 8, 9), if these patients hadn't undergone surgery, they would not have recovered within a short period. So, we propose that when we are aware of ERCP related perforation, emergency operation would be better than delayed operation because of the poor prognosis for cancer leakage as well as high morbidity for delayed operation. It would be best if ERCP is performed under preparation for extensive surgery just in case of suspicious malignancy. Sometimes it is necessary to perform ERCP before completion of preparation for extensive surgery. Using frozen biopsy in cases of suspicious malignancy will help to minimize the extent of operation of transduodenal phincteroplasty or primary closure.
Sarli et al. [16] reported a wide range of operative procedures for the treatment of ERCP-associated perforations, including simple retroperitoneal drainage, duodenal repair around a T-tube inserted into the perforation, common bile duct exploration and T-tube placement, duodenal diversion by antrectomy and gastrojejunostomy or gastrojejunostomy with pyloric exclusion, and pancreaticoduodenectomy. We employed different methods including primary closure, T-tube choledochostomy, duodenal diverticulization, and the classic Whipple operation based on radiologic and operative findings. We believe that the operative modality is best decided on a case-by-case basis.
In conclusion, type I injuries require immediate surgical management after ERCP or immediate endoscopic closure during ERCP. When type II injuries occur, CT evaluation is needed for evaluation of fluid collection. If there is dirty fluid collection in the intra- and retro-peritoneal area, surgical management should be considered. If there is no fluid collection, conservative treatment is possible. Type III injuries almost always require conservative treatment with ENBD drainage.
When surgery is recommended, immediate surgery is preferable to delayed surgery due to high morbidity (Fig. 2).
Figures and Tables
Fig. 1
Classification of endoscopic retrograde cholangiopancreatography-related perforations according to injury mechanism.
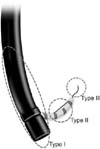
Fig. 2
Algorithm for the management of endoscopic retrograde cholangiopancreatography-related perforations. CT, computed tomography.
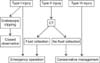
Table 5
Treatment of type II injuries

ERCP, endoscopic retrograde cholangiopancreatography; R/O, rule out; IPMN, intraductal papillary mucinous neoplasm; CBD, common bile duct; Abd., abdominal; CT, computed tomography; PPPD, pylorus preserving pancreaticoduodenectomy; POD, post-operative days.
a)Patient no. 6 This patient was transfer to other hospital at the request of the patient after 4 days of conservative management.
References
1. Christensen M, Matzen P, Schulze S, Rosenberg J. Complications of ERCP: a prospective study. Gastrointest Endosc. 2004. 60:721–731.
2. Enns R, Eloubeidi MA, Mergener K, Jowell PS, Branch MS, Pappas TM, et al. ERCP-related perforations: risk factors and management. Endoscopy. 2002. 34:293–298.
3. Pungpapong S, Kongkam P, Rerknimitr R, Kullavanijaya P. Experience on endoscopic retrograde cholangiopancreatography at tertiary referral center in Thailand: risks and complications. J Med Assoc Thai. 2005. 88:238–246.
4. Booth FV, Doerr RJ, Khalafi RS, Luchette FA, Flint LM Jr. Surgical management of complications of endoscopic sphincterotomy with precut papillotomy. Am J Surg. 1990. 159:132–135.
5. Stapfer M, Selby RR, Stain SC, Katkhouda N, Parekh D, Jabbour N, et al. Management of duodenal perforation after endoscopic retrograde cholangiopancreatography and sphincterotomy. Ann Surg. 2000. 232:191–198.
6. Howard TJ, Tan T, Lehman GA, Sherman S, Madura JA, Fogel E, et al. Classification and management of perforations complicating endoscopic sphincterotomy. Surgery. 1999. 126:658–663.
7. Kayhan B, Akdoğan M, Sahin B. ERCP subsequent to retroperitoneal perforation caused by endoscopic sphincterotomy. Gastrointest Endosc. 2004. 60:833–835.
8. Morgan KA, Fontenot BB, Ruddy JM, Mickey S, Adams DB. Endoscopic retrograde cholangiopancreatography gut perforations: when to wait! When to operate! Am Surg. 2009. 75:477–483.
9. Seibert DG. Use of an endoscopic clipping device to repair a duodenal perforation. Endoscopy. 2003. 35:189.
10. Mutignani M, Iacopini F, Dokas S, Larghi A, Familiari P, Tringali A, et al. Successful endoscopic closure of a lateral duodenal perforation at ERCP with fibrin glue. Gastrointest Endosc. 2006. 63:725–727.
11. Ryozawa S, Iwamoto S, Iwano H, Ishigaki N, Taba K, Sakaida I. ERCP using double-balloon endoscopes in patients with Roux-en-Y anastomosis. J Hepatobiliary Pancreat Surg. 2009. 16:613–617.
12. Husain S, Garmager K, McPhee MS, Jacob KM, Fisher JK, Helzberg JH. The significance of retroperitoneal air following endoscopic sphincterotomy [abstract]. Gastrointest Endosc. 1995. 41:400.
13. Cho MS, Park DE, Chae KM. Management for duodenal perforation caused by endoscopic retrograde cholangiopancreatography (ERCP). J Korean Surg Soc. 2007. 72:210–215.
14. Chung RS, Sivak MV, Ferguson DR. Surgical decisions in the management of duodenal perforation complicating endoscopic sphincterotomy. Am J Surg. 1993. 165:700–703.
15. Avgerinos DV, Llaguna OH, Lo AY, Voli J, Leitman IM. Management of endoscopic retrograde cholangiopancreatography: related duodenal perforations. Surg Endosc. 2009. 23:833–838.
16. Sarli L, Porrini C, Costi R, Regina G, Violi V, Ferro M, et al. Operative treatment of periampullary retroperitoneal perforation complicating endoscopic sphincterotomy. Surgery. 2007. 142:26–32.




 ePub
ePub Citation
Citation Print
Print







 XML Download
XML Download