Dear Editor,
Aerococcus viridans is a catalase-negative gram-positive coccus that appears in clusters, tetrads, or irregular arrangements [1]. This organism is generally considered as a contaminant in clinical cultures, but is also infrequently reported as a clinically significant isolate that causes endocarditis, bacteremia, spondylodiscitis, and urinary tract infections [2345]. Although most A. viridans strains were susceptible to penicillin and other commonly used antibiotics, Uh et al [6] described a case of bacteremia caused by an A. viridans strain showing high resistance to penicillin, erythromycin, clindamycin, and ceftriaxone. In 2014, Zhou et al [7] reported a peritoneal dialysis-related infection caused by vancomycin-resistant A. viridans harboring the vanA gene. We report a case of a vancomycin-resistant A. viridans isolate obtained from an excisional biopsy wound. As far as we know, no study regarding vancomycin-resistant A. viridans has yet been published in Korea.
A 77-yr-old farmer visited the emergency room complaining of severe chills. Swelling in an external wound was observed on the inguinal areas where previous excisional biopsy was performed. He had recently been diagnosed as having colon adenocarcinoma and angioimmunoblastic T-cell lymphoma. On admission, his body temperature was 39.0℃. Hematological investigation revealed a hemoglobin level of 9.2 g/dL, white blood cell (WBC) count of 18.96×109/L (segmented neutrophils; 92.2%), and platelet count of 262×109/L. Serum C-reactive protein (CRP) level (14.36 mg/dL, reference range: <0.30 mg/dL) was elevated. Two aerobic and anaerobic blood culture sets were incubated in the BacT/Alert 3D system (bioMérieux, Durham, NC, USA). The mucus-like whitish aspirate from the wound was plated onto 5% sheep blood agar (BD Diagnostic Systems, Sparks, MD, USA), MacConkey agar (BD Diagnostic Systems), and fluid thioglycollate medium (BD Diagnostic Systems).
Some colonies grew on the 5% sheep blood agar after 24 hr aerobic incubation at 35℃; these colonies were identified as oxacillin-resistant Staphylococcus hemolyticus by VITEK 2 (bioMérieux, Marcy l'Etoile, France). No growth was detected in the blood cultures after five days of incubation. Thirteen days after hospital admission, chemotherapy for lymphoma was initiated. Although WBC count and CRP level were within reference ranges at that time, mucus-like discharge from the wound remained, and CRP started to rise steadily. A. viridans and S. hemolyticus were repeatedly simultaneously isolated from wound cultures. The identification probability of A. viridans by VITEK 2 was 98%. Antimicrobial susceptibility test by a MicroScan MICroSTREP plus panel (Beckman Coulter, Brea, CA, USA) showed that A. viridans was susceptible to tetracycline, but resistant to vancomycin, penicillin, cefotaxime, ceftriaxone, sulfamethoxazole/trimethoprim, meropenem, and levofloxacin.
To confirm the species identification and antimicrobial susceptibilities, 16S rRNA sequence analysis, minimal inhibitory concentration (MIC) determination for vancomycin (Daewoong Lilly, Seoul, Korea) and teicoplanin (Narion Merrell Dow, Seoul, Korea), and PCR to detect vanA and vanB genes were performed. MICs were performed by using the broth microdilution method according to CLSI guidelines [8]. PCR amplification of 16S rRNA was performed by using primers 16SF (5′-TAA YAC ATG CAA GTC GAR CG-3′) and 608R (5′-TAT TAC CGC GGC TGC TGG CA-3′), and sequencing was conducted by using the Big Dye Terminator Cycle Sequencing kit (Applied Biosystems, Foster City, CA, USA) and an ABI PRISM 3730 genetic analyzer (Applied Biosystems). All sequences were analyzed by using the basic local alignment search tool and ribosomal database project. The 450-bp 16S rRNA gene sequence from our isolate showed 100% similarity to and 99% query coverage with several A. viridans strains (GenBank accession no. KR140225.1, LN998006.1, EU169542.1). The primers and PCR procedure for amplification of vanA and vanB genes were previously described [9]. Each DNA product was tested by using gel electrophoresis, where vanA gene was conclusively detected. A. viridans showed a high degree of resistance to vancomycin (<128 µg/mL) and teicoplanin (64 µg/mL), indicating potential acquisition of the vanA gene.
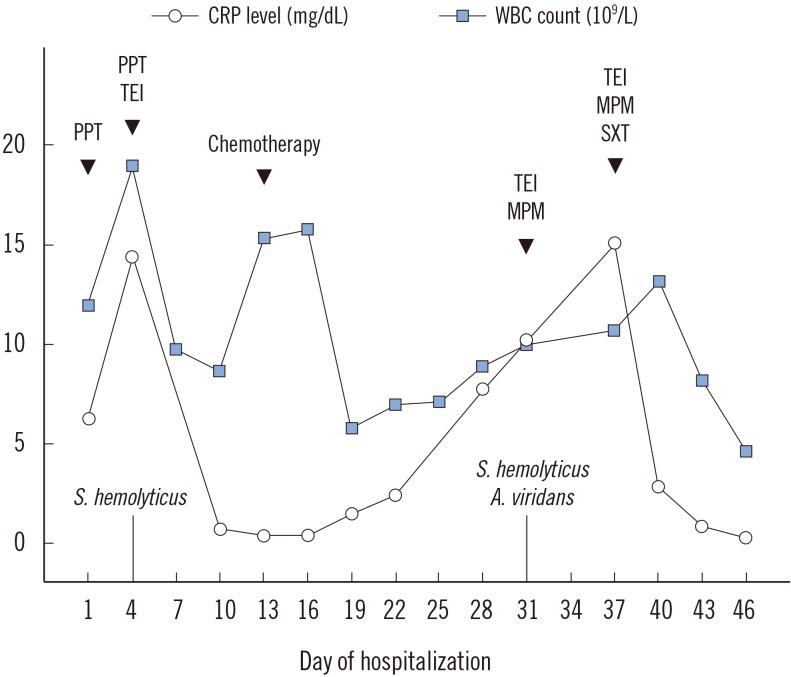
The physician added sulfamethoxazole/trimethoprim to teicoplanin and meropenem combination therapy. Consequently, exudate lessened, and WBC count and CRP level also decreased (Fig. 1).
It is difficult to distinguish simple colonization from real infection when A. viridans is identified in a wound specimen. However, we paid attention to our A. viridans isolate because this patient struggled with chemotherapy before A. viridans was isolated and because the resistance may spread to other gram-positive cocci through transfer of vanA from A. viridans [10].
References
1. Rasmussen M. Aerococci and aerococcal infections. J Infect. 2013; 66:467–474. PMID: 23277106.
2. Moreno LZ, Matajira CE, Gomes VT, Silva AP, Mesquita RE, Christ AP, et al. Molecular and antibiotic susceptibility characterization of Aerococcus viridans isolated from porcine urinary infection. Vet Microbiol. 2016; 184:7–10. PMID: 26854338.
3. Kerbaugh MA, Evans JB. Aerococcus viridans in the hospital environment. Appl Microbiol. 1968; 16:519–523. PMID: 5649865.
4. Gopalachar A, Akins RL, Davis WR, Siddiqui AA. Urinary tract infection caused by Aerococcus viridans, a case report. Med Sci Monit. 2004; 10:CS73–CS75. PMID: 15507858.
5. Popescu GA, Benea E, Mitache E, Piper C, Horstkotte D. An unusual bacterium, Aerococcus viridans, and four cases of infective endocarditis. J Heart Valve Dis. 2005; 14:317–319. PMID: 15974524.
6. Uh Y, Son JS, Jang IH, Yoon KJ, Hong SK. Penicillin-resistant Aerococcus viridans bacteremia associated with granulocytopenia. J Korean Med Sci. 2002; 17:113–115. PMID: 11850599.
7. Zhou WQ, Niu DM, Zhang ZZ, Ning MZ, Shen H, Zhang K. Vancomycin resistance due to VanA in an Aerococcus viridans isolate. Indian J Med Microbiol. 2014; 32:462–465. PMID: 25297044.
8. Clinical and Laboratory Standards Institute. Methods for antimicrobial dilution and disk susceptibility testing of infrequently isolated or fastidious bacteria. CLSI guideline M45. Wayne, PA: Clinical and Laboratory Standards Institute;2015.
9. Kwon OG, Uh Y, Jang IH, Lee MK, Yoon KJ, Kim HY. Trend of isolation and genotypes of vancomycin-resistant enterococci isolated from tertiary care hospital in Wonju area. Korean J Clin Pathol. 2000; 20:486–493.
10. Périchon B, Courvalin P. VanA-type vancomycin-resistant Staphylococcus aureus. Antimicrob Agents Chemother. 2009; 53:4580–4587. PMID: 19506057.




 PDF
PDF ePub
ePub Citation
Citation Print
Print


 XML Download
XML Download