Dear Editor
Brachydactyly is a heterogeneous group of inherited disorders of the digits, characterized by shortened fingers and/or toes [1]. Brachydactyly can occur as an isolated feature or as a part of a complex malformation syndrome [2]. Isolated brachydactylies have been classified into five types, A through E, on the basis of digit malformation. Among them, brachydactyly type A1 (BDA1; MIM 112500) is characterized by bilaterally symmetrical shortening or absence of the middle phalanges of most digits [1].
BDA1 is a rare hand malformation with few reported pedigrees. The phenotypic variability of BDA1 has been documented in familial cases and includes additional features such as other hand/foot malformations, scoliosis, and short stature [1]. Genes associated with BDA1 include IHH [3], BDA1B [4], and GDF5 [5]. To our knowledge, no BDA1 case has been confirmed by molecular genetic analysis in the Korean population. Herein, we report a Korean BDA1 patient with IHH mutation.
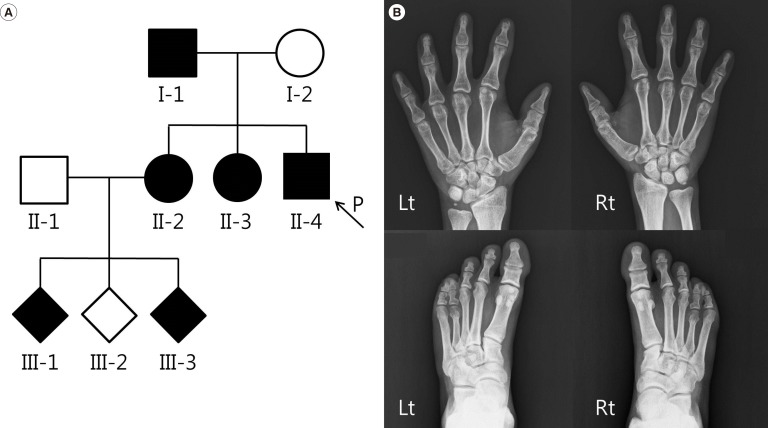
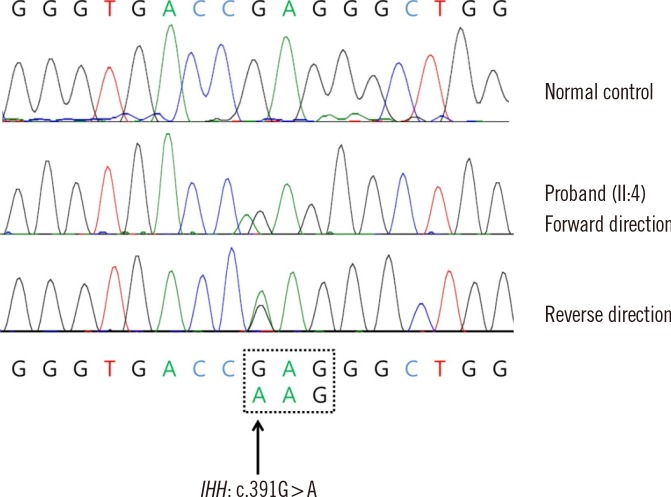
A 32-yr-old man with shortening of digits on both hands and feet presented to our hospital for evaluation. He was of normal stature and development compared with general Korean standards. His prenatal history was unremarkable. The range of motion of his hand joints was normal, and he had no other skeletal or non-skeletal dysmorphisms. The proband's father (I-1) and his two sisters (II-2 and II-3) also had brachydactyly (Fig. 1A). The first sister had two affected children (III-1 and III-3) (Fig. 1A). Radiographs of the proband showed the absence of the 2nd-5th middle phalanges and hypoplastic proximal phalanges in both hands and feet (Fig. 1B), characteristic of BDA1. Although genetic heterogeneity of BDA1 has been documented, most of the reported affected families carried a mutation in the IHH gene; thus, we analyzed the IHH gene sequence. Informed consent was obtained, and whole blood was collected from the patient, in EDTA vacutainer. Genomic DNA was extracted from peripheral blood leukocytes, and all three coding exons were amplified by PCR using primers that we designed (available on request). PCR products were sequenced on an ABI 3730×lDNA Analyzer (Applied Biosystems, Foster City, CA, USA) by using a BigDye Terminator v3.1 Cycle sequencing kit (Applied Biosystems). Sequences were analyzed by using Sequencher software (Gene Codes Corp., Ann Arbor, MI, USA) and compared with the reference sequence for IHH (NM_002181.3). A missense mutation in the IHH gene (c.391G>A; p.Glu131Lys) was identified in the patient (Fig. 2). Other affected individuals (I-1, II-2, II-3, III-1, and III-3) refused the genetic test; thus, a family study could not be conducted in this case.
This is the first genetically confirmed case of BDA1 in the Korean population. The p.Glu131Lys mutation has been previously described in two families of Ashkenazi Jewish and Chinese ethnic origins [3, 6, 7]. Affected individuals of the Ashkenazi Jewish families were reported to be missing the middle phalanges in the 2nd-5th digits, as in our patient [6]. This phenotype, which is common to both families, suggests a particularly important role of Glu131 in IHH gene function throughout skeletal development. In the Chinese family with the p.Glu131Lys variant, the involved brachydactyly sites varied among the different family members as did other hand anomalies such as shortened proximal phalanges of the thumbs and clinodactyly, which suggests the phenotypic variability of BDA1 [3, 7].
Most of the reported BDA1 families have a mutation in the IHH gene localized to chromosome 2q33-q35. The Ihh gene, which encodes a member of the Hedgehog family of secreted signaling molecules, is known for its role in mediating condensation, growth, and differentiation of long bone cartilage templates [8]. In an experiment involving Ihh -/- mice, loss of Ihh signaling resulted in a limb reduction phenotype, with a complete lack of osteoblast development in all bones that develop by endochondral ossification, highlighting the role played by Ihh in cartilage differentiation and bone formation [9]. To date, 10 different missense mutations and one deletion have been identified in the Human Gene Mutation Database (HGMD, 2014.3). Almost all BDA1-causing IHH mutations are missense mutations and are restricted to the N-terminal active fragment spanning codons 95-154 [6]. p.Glu131Lys mutation located in the N-terminal fragment affects Hedgehog binding to the receptor Patched1, reducing its capacity to induce cellular differentiation [10].
In conclusion, we identified p.Glu131Lys mutation in the IHH gene. To our knowledge, this is the first genetically confirmed case of BDA1 in the Korean population. This report will contribute to a better understanding of the genetic background of Korean BDA1 patients.
References
2. Utine GE, Breckpot J, Thienpont B, Alanay Y, Aksoy C, Boduroğlu K, et al. A second patient with Tsukahara syndrome: type A1 brachydactyly, short stature, hearing loss, microcephaly, mental retardation and ptosis. Am J Med Genet A. 2010; 152A:947–949. PMID: 20358606.

3. Gao B, Hu J, Stricker S, Cheung M, Ma G, Law KF, et al. A mutation in Ihh that causes digit abnormalities alters its signalling capacity and range. Nature. 2009; 458:1196–1200. PMID: 19252479.

4. Armour CM, McCready ME, Baig A, Hunter AG, Bulman DE. A novel locus for brachydactyly type A1 on chromosome 5p13.3-p13.2. J Med Genet. 2002; 39:186–188. PMID: 11897820.

5. Byrnes AM, Racacho L, Nikkel SM, Xiao F, MacDonald H, Underhill TM, et al. Mutations in GDF5 presenting as semidominant brachydactyly A1. Hum Mutat. 2010; 31:1155–1162. PMID: 20683927.

6. Byrnes AM, Racacho L, Grimsey A, Hudgins L, Kwan AC, Sangalli M, et al. Brachydactyly A-1 mutations restricted to the central region of the N-terminal active fragment of Indian Hedgehog. Eur J Hum Genet. 2009; 17:1112–1120. PMID: 19277064.

7. Yang X, She C, Guo J, Yu AC, Lu Y, Shi X, et al. A locus for brachydactyly type A-1 maps to chromosome 2q35-q36. Am J Hum Genet. 2000; 66:892–903. PMID: 10712204.

8. Vortkamp A, Lee K, Lanske B, Segre GV, Kronenberg HM, Tabin CJ. Regulation of rate of cartilage differentiation by Indian hedgehog and PTH-related protein. Science. 1996; 273:613–622. PMID: 8662546.

9. St-Jacques B, Hammerschmidt M, McMahon AP. Indian hedgehog signaling regulates proliferation and differentiation of chondrocytes and is essential for bone formation. Genes Dev. 1999; 13:2072–2086. PMID: 10465785.

10. Ma G, Yu J, Xiao Y, Chan D, Gao B, Hu J, et al. Indian hedgehog mutations causing brachydactyly type A1 impair Hedgehog signal transduction at multiple levels. Cell Res. 2011; 21:1343–1357. PMID: 21537345.





 PDF
PDF ePub
ePub Citation
Citation Print
Print


 XML Download
XML Download