Abstract
Objective
The objective of this study is to evaluate the clinical presentation and outcomes of patients with an intracerebral hematoma (ICH) associated with a ruptured middle cerebral artery (MCA) aneurysm, and the correlation factors associated with the aneurysm and characteristics of the hematoma.
Methods
A retrospective evaluation of clinical and radiologic characteristics and outcomes was conducted for 24 patients (11 men and 13 women; mean age, 53 years) with ruptured MCA aneurysms associated with ICH between September 2008 and December 2011.
Results
Thirteen (54%) of the 24 patients had a favorable outcome, four (17%) suffered from severe disability, and seven (29%) died. Based on Hunt and Hess grade, one patient was classified as Grade II, three as Grade III, 12 as Grade IV, and eight as Grade V. Patients with an unfavorable outcome had significantly larger aneurysms (p = 0.047) and ICH volumes (p = 0.002), compared with patients in the group with a favorable outcome. The most frequent rupture point of aneurysms was the lateral aspect of the aneurysm (54.2%). When the rupture point is toward the lateral direction, the distribution of ICH tended to be located at the temporal lobe and intrasylvian.
Conclusion
Results of the present study suggest an association of the initial clinical state, the size of the aneurysm, and ICH volume with outcome. Although no difference was observed between the location of the rupture point and patient outcomes, an accurate assessment of ICH patterns and the rupture point in angiography may help to ensure surgical exposure and a safe aneurysm clipping.
An intracerebral hematoma (ICH) is described in 4% to 42.6% of cases involving simultaneous occurrence of subarachnoid hemorrhages (SAH) from ruptured cerebral aneurysms.1) The middle cerebral artery (MCA) aneurysms, responsible for up to 35-55% of all aneurysm-related hematoma, are the aneurysms most frequently associated with ICH.2) In these circumstances, immediate workup including a computed tomographic angiography (CTA) or angiographic evaluation is required. This is followed by an urgent decompressive craniectomy, and, due to a rapid deterioration and a high fatality rate, hematoma evacuation with aneurysm clipping or one-stage coil embolization (in patients in whom clinical status permits coiling),3) followed by a craniectomy to evacuate the hematoma should be performed.4-7) Even with an aneurysm clipping and hematoma aspiration, the reported mortality rate ranges from 21% to 85%.2)6)8-11) Some authors have reported that poor-grade patients with ICH at admission who underwent early surgical treatment showed results similar to those of patients without ICH, and have suggested that ICH associated with a ruptured aneurysm is not associated with a poorer final prognosis if early surgical treatment is performed.8)12)13)
We report here on our experiences with a series of 24 ICH patients associated with ruptured MCA aneurysms, and the prognostic factors correlated with the characteristics of the aneurysm and hematoma.
From September 2008 to December 2011, 105 patients with ruptured MCA aneurysms were treated with microsurgical clipping or coil embolization at our institution. All patients met the following inclusion criteria: (a) presentation of the condition within the first 12 hours, (b) computed tomography (CT) evidence of SAH associated with an ICH, and (C) CTA or angiographic evidence of an MCA aneurysm. Exclusion criteria were as follows: (a) definitively dissecting aneurysm, (b) therapeutic anticoagulation, and (C) a Glasgow Coma Scale (GCS) score of 3 without spontaneous respirations.
Of these patients, 35 patients with ICH caused by a ruptured MCA aneurysm from our retrospectively collected database of patients were reviewed. In total, 11 patients were excluded because of M2 dissecting aneurysms treated with conservative management (n = 1), therapeutic anticoagulation (n = 4), and GCS score of 3 without spontaneous respiration (n = 4). Accordingly, 24 patients were identified and enrolled; 11 (45.8%) males and 13 (54.2%) females, ranging in age from 36 to 72 years (mean 53.3 ± 10.07 years). Patients were graded according to the Hunt and Hess grade (HHG) at admission and pretreatment. After six months, to assess the outcome, the Glasgow Outcome Scale (GOS) was used for classification of each patient as having either a good recovery and moderate disability (a favorable outcome), or a severe disability, a vegetative state, or death (an unfavorable outcome).
Noncontrast enhanced CT scans and CTA were reviewed for hematomas, defined as a collection of blood with a diameter of ≥ 3 cm. The localization of the hematoma was determined on the basis of criteria defined in previous studies:14)15) 1) an intra-sylvian hematoma based on the sylvian fissure bleeding pattern; 2) a temporal ICH; 3) a frontal ICH. The volume of hematoma was calculated using a modified ellipsoid volume formula (ABC/2 method).16)
On admission, CTA was performed in order to roughly identify the location and configuration of an aneurysm. This was followed by a digital subtraction angiogram (DSA) in patients of all clinical grades, unless the patients were hemodynamically unstable or moribund. The CTA and/or DSA of each patient were examined for M1 elevation, the MCA sylvian point, and the size and rupture point of the aneurysm on the anteroposterior projection.
The treatment decision (clip, coil or combined with hematoma evacuation) was based on patients' situation (age, medical condition, volume of hematoma, figure of aneurysm, etc). Ruptured aneurysms were clipped at the time of ICH evacuation or coiled before the ICH evacuation.
In two patients, one-stage coil embolization was attempted without hematoma evacuation. Their primary indication for coiling was a minor or moderate mass effect of the hematoma and a favorable configuration for endovascular therapy on CTA.
In 22 patients, we performed surgical clipping and hematoma evacuation. For all surgeries, a wider expanded pterional craniotomy or fronto-temporal-parietal craniotomy was performed. After the dura had been incised, we first evacuated some amount of hematoma in order to release brain swelling for prevention of retraction injury and to gain easy access proximal control. After the aneurysm was clipped, the remaining hematoma was evacuated. When the brain swelling persisted and high intracranial pressure (ICP) was expected, duroplasty and/or craniectomy with enlargement of the bone flap were performed and the bone flap was not replaced.
Baseline characteristics including age, sex, clinical status, hematoma type and volume are shown in Table 1. Based on HHG, one patient was classified as Grade II; three as Grade III; 12 as Grade IV; and eight as Grade V. Evaluation of the outcomes according to GOS at 6 months after treatment was as follows: good in five cases, moderate in eight (favorable outcome, 54%), severe or vegetative in four (17%), and death in seven (29%). No significant difference in mean age was observed between the groups with favorable and unfavorable outcomes. Patients with good-grade HHG at pretreatment had the better outcomes (p = 0.014).
Patients with an unfavorable outcome had significantly larger aneurysms, compared with patients in the group with a favorable outcome (mean, 11.5 versus 6.3 mm; p = 0.047). A significant difference in the mean ICH volume was observed between the group showing a favorable (33.2 ± 21 cc) and the group showing an unfavorable outcome (68.7 ± 29.7 cc; p = 0.002).
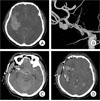
The most frequent rupture point of aneurysms was the lateral aspect of the aneurysm (54.2%). In patients with a superior rupture point, the ICH was more frequently located at the frontal lobe (Fig. 1). When the rupture point is toward the lateral direction, the distribution of ICH tended to be located at the temporal lobe and intrasylvian (Fig. 2). However, in comparison with the ICH volume, the location of the rupture point did not differ significantly between favorable outcome and another. Although there was no difference in prognosis among the locations of ICH, cases with including intrasylvian hematomas tended to have worse prognosis.
Outcomes for patients with ruptured MCA aneurysms with ICH have been suggested to be worse, compared with SAH cases without ICH.2)8)10)14) Previous studies regarding patients having aneurysm rupture with ICH reported unfavorable outcomes for 61 to 88%, and mortality rates of up to 58% because those patients tend to have a more severe clinical grade on admission, severe brain swelling, and high rebleeding rate before aneurysm obliteration.5) The mortality rate after conservative treatment or hematoma evacuation only without clipping is 75 to 80%.10)17)18)
Tokuda et al.10) reported a better outcome for patients having an ICH with a hematoma volume of less than 40 cm3 when a hematoma evacuation and aneurysm obliteration are performed. In our cases, 84.6% of patients with a hematoma volume of less than 40 cm3 had favorable outcomes.
To prevent retraction injury and gain easy access proximal control, careful hematoma removal should be performed where the distal portion from the aneurysm. After the aneurysm was clipped, the remaining hematoma was evacuated. When significant brain swelling and edema were encountered, partial lobectomy and craniectomy were performed. In our institution, after evacuation of a hematoma, a silicone catheter is inserted into the residual hematoma space, followed by careful manual hematoma aspiration and connection to the closed external drainage system (Fig. 2D). Then the patients are irrigated with a thrombolytic agent, 3,000 IU urokinase, four times per day for three or five days. Because further evacuation of the residual hematoma by catheter drainage has the benefit of reducing hematoma volume and the chance of edema formation, thus improving cerebral perfusion, it may reduce the incidence of morbidity and mortality.
In our report, although there is no distinct difference between hematoma volume and rupture point of MCA aneurysms, there is an interesting feature of the hematoma location according to the rupture point of the MCA aneurysms. Because almost the whole aneurysm sac is adhered to the pia mater, ICH associated with an aneurysm is located in the subcortical area. ICH associated with a MCA aneurysm is located primarily in the temporal lobe, intrasylvian fissure, external capsule, and lateral frontal base. Anatomically, the outcome for subcortical ICH following evacuation of the ICH by emergency craniotomy is favorable. However, large intrasylvian hematomas and peribrainstem clots can cause direct compression of the brain stem requiring an ultra-early surgical decompression. During removal of an intrasylvian hematoma, careful attention should be paid to prevention of premature aneurysmal rebleeding and vessel injury. In our series, the most frequent rupture point of the MCA aneurysm in intrasylvian ICH was the supero-lateral aspect of the aneurysm sac. We suggest that accurate assessment of bleeding patterns and identification of the rupture point prior to surgical undertaking may assist the surgeon in preventing premature rebleeding, predicting the clinical course, and determining the appropriate treatment.14)
Results of the present study suggest an association of the initial clinical state, the size of the aneurysm, and ICH volume with clinical outcome. The location of the rupture point of the aneurysm may be associated with the ICH location, and, therefore, an accurate assessment of ICH patterns and the rupture point in CTA or DSA may help to ensure appropriate surgical exposure and a safe aneurysm clipping.
Figures and Tables
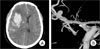
Fig. 1
Admission brain computed tomography (CT) scan shows right frontal intracerebral hemorrhage (ICH) with a mass effect by ipsilateral ventricle compression (A). Right three-dimensional digital subtraction angiogram (3D-DSA) shows the rupture point of the middle cerebral artery (MCA) bifurcation aneurysm projecting superiorly (B).
References
1. Niemann DB, Wills AD, Maartens NF, Kerr RS, Byrne JV, Molyneux AJ. Treatment of intracerebral hematomas caused by aneurysm rupture: coil placement followed by clot evacuation. J Neurosurg. 2003. 11. 99(5):843–847.

2. Guresir E, Beck J, Vatter H, Setzer M, Gerlach R, Seifert V, et al. Subarachnoid hemorrhage and intracerebral hematoma: incidence, prognostic factors, and outcome. Neurosurgery. 2008. 12. 63(6):1088–1093. discussion 1093-4.
3. Shin YS, Kim SY, Kim SH, Ahn YH, Yoon SH, Cho KH, et al. One-stage embolization in patients with acutely ruptured poor-grade aneurysm. Surg Neurol. 2005. 02. 63(2):149–154. discussion 154-5.

4. Bailes JE, Spetzler RF, Hadley MN, Baldwin HZ. Management morbidity and mortality of poor-grade aneurysm patients. J Neurosurg. 1990. 04. 72(4):559–566.

5. Brandt L, Sonesson B, Ljunggren B, Saveland H. Ruptured middle cerebral artery aneurysm with intracerebral hemorrhage in younger patients appearing moribund: emergency operation? Neurosurgery. 1987. 06. 20(6):925–929.

6. Heiskanen O, Poranen A, Kuurne T, Valtonen S, Kaste M. Acute surgery for intracerebral haematomas caused by rupture of an intracranial arterial aneurysm. A prospective randomized study. Acta Neurochir (Wien). 1988. 90(3-4):81–83.
7. Hernesniemi J, Vapalahti M, Niskanen M, Tapaninaho A, Kari A, Luukkonen M, et al. One-year outcome in early aneurysm surgery: a 14 years experience. Acta Neurochir (Wien). 1993. 122(1-2):1–10.

8. Tokuda Y, Inagawa T, Katoh Y, Kumano K, Ohbayashi N, Yoshioka H. Intracerebral hematoma in patients with ruptured cerebral aneurysms. Surg Neurol. 1995. 03. 43(3):272–277.

9. Baskaya MK, Menendez JA, Yuceer N, Polin RS, Nanda A. Results of surgical treatment of intrasylvian hematomas due to ruptured intracranial aneurysms. Clin Neurol Neurosurg. 2001. 04. 103(1):23–28.
10. Shimoda M, Oda S, Mamata Y, Tsugane R, Sato O. Surgical indications in patients with an intracerebral hemorrhage due to ruptured middle cerebral artery aneurysm. J Neurosurg. 1997. 08. 87(2):170–175.

11. Nowak G, Schwachenwald D, Schwachenwald R, Kehler U, Muller H, Arnold H. Intracerebral hematomas caused by aneurysm rupture. Experience with 67 cases. Neurosurg Rev. 1998. 21(1):5–9.

12. Abbed KM, Ogilvy CS. Intracerebral hematoma from aneurysm rupture. Neurosurg Focus. 2003. 10. 15(4):E4.

13. Su CC, Saito K, Nakagawa A, Endo T, Suzuki Y, Shirane R. Clinical outcome following ultra-early operation for patients with intracerebral hematoma from aneurysm rupture--focussing on the massive intra-sylvian type of subarachnoid hemorrhage. Acta Neurochir Suppl. 2002. 82:65–69.
14. Yoshimoto Y, Wakai S, Satoh A, Hirose Y. Intraparenchymal and intrasylvian haematomas secondary to ruptured middle cerebral artery aneurysms: prognostic factors and therapeutic considerations. Br J Neurosurg. 1999. 02. 13(1):18–24.

15. van der Zande JJ, Hendrikse J, Rinkel GJ. CT angiography for differentiation between intracerebral and intra-sylvian hematoma in patients with ruptured middle cerebral artery aneurysms. AJNR Am J Neuroradiol. 2011. 02. 32(2):271–275.

16. Kothari RU, Brott T, Broderick JP, Barsan WG, Sauerbeck LR, Zuccarello M, et al. The ABCs of measuring intracerebral hemorrhage volumes. Stroke. 1996. 08. 27(8):1304–1305.





 PDF
PDF ePub
ePub Citation
Citation Print
Print



 XML Download
XML Download