Abstract
The incidence of urothelial carcinoma of the urinary bladder (bladder cancer) remains high. While other solid organ malignancies have seen significant improvement in morbidity and mortality, there has been little change in bladder cancer mortality in the past few decades. The mortality is mainly driven by muscle invasive bladder cancer, but the cancer burden remains high even in nonmuscle invasive bladder cancer due to high recurrence rates and risk of progression. While apoptosis deregulation has long been an established pathway for cancer progression, nonapoptotic pathways have gained prominence of late. Recent research in the role of autophagy in other malignancies, including its role in treatment resistance, has led to greater interest in the role of autophagy in bladder cancer. Herein, we summarize the literature regarding the role of autophagy in bladder cancer progression and treatment resistance. We address it by systematically reviewing treatment modalities for nonmuscle invasive and muscle invasive bladder cancer.
Urothelial carcinoma (UC) of the urinary bladder, the most common form of bladder cancer, remains the fourth most common malignancy in the United States, and ninth worldwide [123]. Among genitourinary malignancies, it trails only prostate cancer in incidence and mortality [14]. Of patients diagnosed with UC, 70% are nonmuscle invasive (NMIBC) and 30% are muscle invasive bladder cancer (MIBC) at the time of diagnosis [5]. While management of NMIBC requires regular surveillance, repeated transurethral resections (TURs), and use of intravesical agents, it is the subset of patients diagnosed with MIBC or those who progress to MIBC that drive the mortality of this disease.
The oft-cited 50% overall survival at 5 years for MIBC has remained relatively unchanged in 20 years. While management of other cancers has progressed rapidly, the management of bladder cancer remains relatively stagnant [67]. The one major change in management in that time period was the introduction of neoadjuvant platinum-based chemotherapy (NAC) for patients with nonmetastatic MIBC prior to undergoing radical cystectomy. Despite the 7% survival benefit demonstrated by NAC, the overall mortality of bladder cancer has not shifted much in the past two decades. Identifying novel therapies and improving response to current therapies may help improve outcomes for patients with bladder cancer.
Most cancer therapies, such as chemotherapy or radiotherapy, mediate their effect by promoting apoptotic cell death, or programmed cell death. Cancer cells that develop the ability to interfere with those pathways subsequently develop resistance to primary cancer therapies; often, this leads to drug-resistant, more aggressive tumors with worse clinical outcomes. Targeting nonapoptotic forms of programmed cell death may help overcome this resistance. Autophagy is one such pathway.
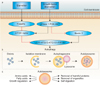
Autophagy is a constitutively active evolutionary conserved physiologic catabolic process that is maintained at a basal rate by cells throughout the body. This means, that under normal healthy environmental conditions, it is not needed and is suppressed. However, under conditions of cell stress, such as hypoxia, radiation or chemotherpy, autophagic activity can be increased significantly to ensure sources of cellular energy. Activation of the autophagy pathway results in sequestration of intracellular proteins and organelles in specialized vacuoles called autophagosomes. These then undergo lysosomal-mediated enzymatic degradation to recycle organic material to maintain metabolism during periods of deprivation [8910]. The process is fluid and consists of sequential steps (initiation, nucleation, elongation, maturation) mediated by a group of autophagy-related genes (ATG genes) [4]. In general, this process helps promote survival in a stress response. Cancer cells utilize this pathway under conditions of metabolic stress induced by different therapies to prolong survival [1112]. The autophagic pathway is shown in Fig. 1 [13].
While the role of autophagy in other malignancies, including prostate adenocarcinoma, has been better studied, the role of autophagy in the progression and treatment of UC is now being established [13141516]. Indeed, a PubMed search for autophagy and bladder cancer resulted in only 70 articles, with all but one published after 2007. However, it is evident based on recent research, that there is a promising role for autophagy focused therapies in the field of bladder cancer.
While 30% of patients have MIBC at the time of initial presentation, there remains a population of patients with NMIBC that progress to muscle invasion despite treatment. NMIBC has a high recurrence rate and approximately 10%–30% ultimately progress to MIBC [17]. Unfortunately, the biology of progression to muscle-invasion is not clearly defined. Recent studies have begun to demonstrate the role of autophagy in UC progression.
Ojha et al. [18] collected tumor tissue samples from 30 patients undergoing TUR of NMIBC, 15 high-grade (HG) and 15 low-grade (LG) based on World Health Organization histopathologic criteria. They compared this against three normal bladder urothelial tissue samples obtained from patients undergoing TUR of prostate for benign prostatic hyperplasia. Using transmission electron microscopy, they demonstrated significantly more autophagic vesicles in the HG UC specimens than in the LG UC specimens; both contained more autophagic vesicles than the benign tissue. Levels of Beclin-1 and Atg7, key proteins associated with autophagosome biogenesis, were increased in HG and LG UC specimens. Conversion of LC3-I to LC3-II, often used as a marker for autophagic induction, was also increased from LG UC to HG UC specimens. Both HG and LG UC were more susceptible to starvation induced autophagy compared to normal urothelial cells. When exposed to autophagy inhibitors, such as wortmannin, 3-methyladenine (3MA), and chloroquine, under similar starvation conditions, there was significantly increased cell death in the HG UC population than in the LG UC population. Caspase-9, an initiator caspase involved in intrinsic apoptotic pathway, was activated in the presence of autophagy inhibitors suggesting apoptosis activation as a mechanism of cell death [18].
Sivridis et al. [19] similarly utilized TUR specimens to attempt to identify immunohistochemical markers that may predict tumor progression. "Stone like" structures (SLS) are physical manifestations of undigested cytoplasmic constituents due to exhaustion of lysosomal enzymes following autophagic cell death, which are readily identified by light microscopy. High numbers of SLS had previously been associated with increased aggressiveness and poorer prognosis in other epithelial malignancies. In this study, 250 TUR specimens were analyzed – 70 LG NMIBC, 70 HG NMIBC, 70 HG MIBC, and 40 controls. LC3 reactivity and SLS concentration was higher in HG NMIBC than in LG MIBC, and more importantly, was significantly higher in MIBC.
More recent work by Lin et al. [20] demonstrated the important of autophagy in bladder cancer progression in known bladder cancer cell lines SV-HUC-1, RT-4, and 5637. Autophagy inhibition with Baf (bafilomycin) A1 or knockdown of Atg-7 resulted in cell death in those same cell lines, due to apoptosis induction.
Baspinar et al. [21] took this one step further. Bcl-1 (Beclin 1) is an important mediator of autophagy. Bcl-1 promotes autophagy, and its suppression sensitizes cells to starvation induced apoptosis. Eighty-four bladder urothelial tumors and 10 normal urothelial specimens from either TUR specimens or final cystectomy specimens were examined, and they identified a significant inverse correlation between Bcl-1 expression and both histologic grade and pT stage (p=0.009, r=–0.284; p=0.001, r=–0.361, respectively). The Bcl-2 family of proteins has oncogenic properties and prevents cell death via antiapoptotic pathways; bcl-2 binds to bcl-1 and inhibits autophagy. As such, they found a significant positive correlation between bcl-2 levels and both histologic grade and pT stage (p=0.026, r=0.243; p<0.0001, r=0.491, respectively) [21]. Shen et al. [22] expanded on this to some degree. They focused their efforts on SMYD3, a histone methyltransferase, which was associated with poor prognosis of patients with prostate and gastric cancer. In their study, they noted that patients with higher SMYD3 expression had poorer prognosis and worse overall survival than patients with lower expression. SMYD3 appears to activate autophagy via upregulation of BCL2-associated transcription factor 1, which in turn is a strong inducer of autophagy. SMYD3 inhibition markedly decreased the activation of autophagy in bladder cancer cell lines as well. As such, they confirmed the importance of the Bcl-2 as demonstrated by Baspinar and colleagues, but also identified a biomarker and potential target for therapy [22]. This provides the clinical correlation between autophagy and UC progression hinted at by the prior studies.
Based on these studies, autophagy clearly plays a role in UC progression. As such, it has some important therapeutic implications. It may serve as an important target for future therapies, and inhibiting its function may help improve response to currently approved therapies.
As mentioned previously, NMIBC represents the majority (70%–80%) of newly diagnosed bladder cancer cases. While, NMIBC itself does not drive bladder cancer mortality, its high risk of recurrence and potential for progression make it a significant area for improvement in the management of bladder cancer. With 50%–80% of patients having recurrences requiring regular surveillance cystoscopies and repeat TURs, the cost burden of NMIBC is also very high. Additionally, 10%–30% of patients with NMIBC eventually progress to MIBC [23].
The current armamentarium utilized by urologists for the management of this disease process includes TUR of bladder tumors, use of intravesical therapies to reduce recurrence and stop progression, and rarely open surgical excision (partial cystectomy or radical cystectomy). Due to the complexity of the management of this disease process, there are multiple published evidence-based guidelines that help guide therapy.
Mitomycin-C is a potent DNA cross-linker chemotherapeutic agent. It been utilized intravesically for NMIBC and demonstrated reduced risk of recurrence but not necessarily progression of UC. It is recommended as a single perioperative dose (or within 24 hours of initial resection) for all bladder tumors or those with presumed LG or intermediate-grade tumors. In addition, it may be used as an adjuvant therapy as weekly instillations in LG tumors [2324].
As has been suggested by some groups, cancer stem cells (CSC) may help drive UC progression. In one study, exposure of primary culture UC cells to mitomycin-C increased the percentage of CSCs in the population. The CSCs also increased expression of glycolytic genes. Inhibition of autophagy using chloroquine resulted in reduced expression of drug resistance genes (MDR1, ABCG2) and also sensitized the CSCs to mitomycin-C induced apoptosis. Use of chloroquine in combination with mitomycin-C may help improve outcomes in LG UC patients [25].
The bacillus Calmette-Guerin (BCG) vaccine is an attenuated live bovine tuberculosis bacillus, Mycobacterium bovis, utilized internationally as a form of tuberculosis vaccination. In the setting of bladder cancer, it has been used as potent immunotherapy.
All published guidelines recommend use of adjunctive intravesical BCG in HG Ta, T1 and carcinoma in situ (CIS) tumors, though the specifics of the administration vary to some degree. The European Association of Urology and American Urologic Association guidelines advocate the longest maintenance schedule of up to 3 years [232627].
With intravesical BCG being a cornerstone of therapy for HG NMIBC, and this population being at highest risk for progression to MIBC, therapies to help improve BCG efficacy carry great potential to affect bladder cancer outcomes and reduce need for extirpative surgeries.
While the mechanism of BCG efficacy is not completely understood, it is thought to generate a local immune reaction, which simultaneously affects NMIBC. As mentioned in Buffen et al. [28], recent literature suggests a process called trained immunity, an epigenetic reprogramming of monocytes. Specifically, monocytes cells that had been previously exposed to BCG were then exposed to Borrelia burgdorferi after a six day rest period; cytokine release was measured after the second exposure was measured. Monocytes previously exposed to BCG had a much higher cytokine release after the B. burgdorferi exposure than those pretreated with placebo. They then demonstrated that autophagy inhibitors 3MA or wortmannin blocked trained immunity; monocytes pretreated with BCG as an initial exposure no longer mounted a significant cytokine response when exposed to B. burgdorferi in the presence of autophagy inhibitors. Specific single nucleotide polymorphisms were identified in autophagy-related genes Atg2B and Atg5 that negatively influenced trained immunity in response to BCG, which correlated to a decrease in autophagosome production in the monocytes. More importantly, they found that in a cohort of 192 NMIBC patients treated with at least six instillations of BCG (equivalent to an induction course), patients that carry one or two C alleles for the rs3759601 Atg2B mutation showed increased risk of recurrence and progression [28].
Improving response to BCG therapy, or even predicting those will not respond, can have significant consequences for management strategies for NMIBC.
Following BCG failure, the standard of care remains radical cystectomy [2627]. In terms of alternative therapies, valrubicin is the only therapy approved in the United States for intravesical salvage therapy, specifically for BCG refractory CIS [329]. Other intravesical agents have been studied for the treatment of NMIBC, and while not recommended for primary treatment, the guidelines do allow for their consideration in the setting of BCG failure. At this time, all utilization is at the discretion of the physician and in discussion with the patient.
Despite that, there has been some work with these agents and autophagy modulation.
While interferon alone has been demonstrated to have some efficacy as a single agent in patients with BCG failure [30], it has more often been studied in combination with BCG [3132]. Benedict et al. [33] had previously report that treatment with adenoviral-mediated interferon α (Ad-IFNα) treatment can overcome interferon resistance. In a more recent study, Zhang et al. [34] demonstrate that treatment with Ad-IFNα mediates an autophagy response, and increased cytotoxicity can be achieved with autophagy inhibition using 3MA.
In some more recent studies, the use of intravesical gemcitabine in isolation or in combination with mitomycin-C has been described for patients with BCG failure, with promising results [33035]. In the study by Ojha et al. [25] previously mentioned, use of chloroquine as an autophagy inhibitor had similar effects on a gemcitabine treated cell lines as for mitomycin-C treated cell lines. It reduced expression of drug resistance genes (MDR1, ABCG2) and also sensitized the CSCs to gemcitabine induced apoptosis. Since the combination gemcitabine/mitomycin-C is now being described for BCG failure patients, the additional an autophagy inhibitor could be synergistic.
Epirubicin, which was found to be inferior in direct comparison with BCG for patients with intermediate or high risk NMIBC especially in setting of BCG maintenance therapy [24], may have a role in combined therapy with BCG based on smaller series. One study described a novel agent, icaritin, a flavonol glycoside, that had cytotoxic efficacy on BT5637 and T24 bladder cancer cells in a time and dose dependent manner; more importantly though, it was found to work synergistically with epirubicin on those same cell lines. Its mechanism was thought to be mediated by inhibiting epirubicin mediated autophagy [36]. Similar results were seen when assessing pirarubicin, another anthracycline described for the treatment of bladder cancer. Autophagy inhibition with 3MA or hydroxychloroquine significantly increased apoptosis in pirarubicin-treated bladder cancer cells, and pirarubicin-induced autophagy was mediated via the mammalian target of rapamycin (mTOR)/p70S6K/4E-BP1 signaling pathway. Knockdown of Atg3 generated similar results [37].
Photodynamic therapy is an emerging modality for treatment of patients with BCG failure. It involves administration of a photosensitizing agent, such as 5-aminolevulinic acid, hexaminolevulinate (HAL), or radachlorin, with activation of the agent by light at the appropriate wavelength. While current studies have mostly been smaller and generally underpowered, there is a promise for future increased utilization [3]. In an effort to help elucidate mechanisms of action of photodynamic therapy with HAL, one group utilized a rat xenograft model and subsequent electron microscopy to identify changes after therapy. They identified significant increased appearance of vacuoles and lipofuscin bodies in the cytoplasm following treatment, which they attributed to increased autophagy [38]. While further work is needed in this area, clearly autophagy plays a role in many different treatment modalities of bladder cancer.
Thirty percent of newly diagnosed bladder cancer patients present with MIBC. In additional, as much as 30% of patients with NMIBC progress to muscle-invasion. As touched on earlier, it is the progression to muscle invasion that is the driver behind the mortality of bladder cancer. Until approximately 15 years ago, the standard of care remained surgery, specifically radical cystectomy [39].
With the development of platinum-based chemotherapy, the first major change in treatment paradigm was introduced [40]. Since its introduction, neoadjuvant chemotherapy has been incorporated into all major international guidelines based on the strength of its data. The 2 major regimens utilized are either MVAC (methotrexate, vinblastine, doxorubicin and cisplatin) or GC (gemcitabine and cisplatin); recent studies have been utilizing a dose-dense MVAC regimen which shortens the duration of treatment. While the specific regimen may vary, platinum based neoadjuvant chemotherapy is now a standard of care [63941].
Bladder sparing therapies have never been demonstrated to be equivalent to cystectomy in this patient population. Despite accepted methodological flaws, all currently published studies have demonstrated that they have worse oncologic outcomes and poorer prognosis. As such, they are not recommended for patients eligible for neoadjuvant chemotherapy and radical cystectomy. However, in certain patients, either due to patient preference or poor candidacy for radical cystectomy, bladder sparing options are utilized. These options range from external beam radiation therapy (EBRT) with or without chemotherapy, partial cystectomy, and maximal TUR resections [41].
As can be expected, a significant portion of the research associated with autophagy in bladder cancer has focused on its potential relationship with chemotherapeutic agents.
Ojha et al. [18] demonstrated that use of chloroquine, the antimalarial drug and a known autophagy inhibitor, augmented cisplatin mediated cytotoxicity in T24 cells without affecting normal utothelial cells. Mani et al. [4] took this one step further. They utilized chemosensitive human bladder cancer lines 5637 and RT4, then developed chemoresistant cell lines through continuous increasing exposure to gemcitabine or cisplatin. They utilized the pan Bcl-2 inhibitor (-)-gossypol to demonstrate that the chemotherapy-resistant cell lines utilized autophagy to escape apoptotic cell death; indeed, they note that overexpression of Bcl-2 proteins has been associated with poor chemotherapeutic response in bladder cancer. Administration of 3MA significantly increased (-)-gossypol mediated cytotoxicity in those same drug-resistant cell lines. Increased LC3 conversion and presence of autophagosomes were identified in the chemoresistant strains compared to the chemosensitive strains; treatment with (-)-gossypol induced even LC3 conversion and autophagosome production [4].
Sepantronium bromide (YM155), a novel small molecule that inhibits survivin, a known pro-apoptotic protein overexpressed in urothelial cancer, was the focus of another group. While they primarily established that YM155 had significant cytotoxic efficacy in the gemcitabine resistant bladder cancer lines, they did also demonstrate that YM155 mediates significant LC3 conversion independently, suggesting its efficacy is in part mediated by autophagy activation. Coadministration of 3-methyladenenine interestingly reducted YM155 mediated cytotoxicity, which the authors contributed to the reduced autophagy mediated cell death due to autophagy inhibition by 3MA. Regardless, this is a promising agent under clinical trial [42].
Li et al. [43] identified a fungal immunomodulatory protein, ganoderma tsugae (FIP-gts), that possesses antitumor activity against solid tumors and inhibits telomerase activity. When utilized to treat UC cell lines, FIP-gts activated LC-3 II conversion, and interestingly, FIP-gts and Baf-A1 combined treatment was found to lead to enhancement of apoptosis along with inhibition of autophagy in chemosensitive and chemoresistant UC cells. They also demonstrated in an earlier study that the dual phosphatidylinositol 3-kinase (PI3K) and mTOR inhibitor NVP-BEZ235 inhibits UC cell growth by activating autophagic flux; it increased production of acidic vesicular organelle development but did not increase LC3 conversion. Of note, administration of chloraquine counteracted the effect of NVP-BEZ235, demonstrating that the anticancer efficacy of NVP-BEZ235 is due to autophagic flux and cotreatment with chloroquine counteracts the cytotoxic effect [44]. This reminds us that autophagy is an important consideration for all future novel therapeutic agents in development as well as those currently approved.
MicroRNAs (miRNAs) have been gaining recognition in their role as regulators of proliferation, migration, invasion and apoptosis in cancer cells. Previously, micro-RNA-222 (miR-222) up-regulation had been noted to be associated with a poor prognosis in bladder cancer [45]. Overexpression of miR-222 significantly inhibited cisplatin mediated cytotoxicity in T24 and 5637 UC cell lines. miR-222 modulates the PPP2R2A/Akt/mTOR pathway in UC cells, and mTOR is a known regulator of autophagy. miR-222 overexpression dramatically reduced cisplatin-mediated autophagy in both UC cell lines, demonstrated by the decreased LC3-II levels, thereby indicating that miR-222 might block cisplatin-induced autophagy in bladder cancer cells. As such, miR-222 may present a viable future target for therapy and help resensitive cells to cisplatin treatment [46].
The mTOR pathway is a regulator of the autophagic pathway. Pinto-Leite et al. [47] utilized this fact to assess the efficacy of temsirolimus, a known mTOR inhibitor used for the treatment of other solid tumors, in conjunction with gemcitabine and cisplatin to 5637, T24, and HT1376 bladder cancer cell lines. Temsirolimus, in isolation, caused mild reduction in proliferation in a three cell lines, but in combination with gemcitabine or cisplatin, the effect was much more pronounced. Though the increase in autophagic cells was mainly pronounced in the temsirolimus and cisplatin combination arm, there was a suggestion of autophagy induction and contribution to improved cytotoxicity [47].
These studies demonstrate the further research into the autophagy process can not only improve response to current therapies, but they can provide new targets for future therapies and potentially find new indications for previously known agents.
Although utilized mainly in bladder sparing regimens, and often in conjunction with chemotherapy, EBRT does have has a role in the management of MIBC [4148]. A method to better select patients who will respond to EBRT may help change guidelines for MIBC management. One group looked specifically at HMGB1 (High Mobility Group Box protein 1) as a potential predictive marker for EBRT response in MIBC. They identified that higher levels of HMGB1 correlated with radiotherapy resistance. Indeed, with HMGB1 knockdown, >1.5-fold sensitization to radiation therapy was achieved, and autophagy was inhibited more than 3 folds (as measured by LC3 conversion). HMGB1 knockdown tumors in their mouse xenograft model showed a significantly better response to radiation therapy and decreased autophagy as compared to controls [49]. This potentially can be a very promising predictive marker and even potential target for patients undergoing EBRT.
While the management and morbidity of bladder cancer has remained relatively stagnant for the past few decades without any significant improvement in outcomes, there has been an increasing effort to identify new methods of treatment. Autophagy, which has been demonstrated to have an important role in treatment resistance in other malignancies, is gaining more recognition for its role in bladder cancer. At all stages and for all modalities of bladder cancer treatment, autophagy has been demonstrated to contribute to treatment resistance. Targeting autophagy may help augment current treatments and develop novel therapies.
Figures and Tables
Fig. 1
Starvation and growth-factor deprivation stimulates cell signaling cascades (the key intermediates identified above) that ultimately result in the initiation of autophagy. The autophagosome matures and is trafficked to lysosomes where it fuses and creates the autolysosome. The autolysosome digests cellular biomass and eliminates toxins, facilitating a host of favorable nutrient and growth conditions. AMPK, AMP-activated protein kinase; mTOR, mechanistic target of rapamycin. Adapted from Farrow JM, et al. Nat Rev Urol 2014;11:508-16, with permission from author C.P.E. [13].
References
1. Siegel RL, Miller KD, Jemal A. Cancer statistics, 2016. CA Cancer J Clin. 2016; 66:7–30.
2. Ferlay J, Shin HR, Bray F, Forman D, Mathers C, Parkin DM. Estimates of worldwide burden of cancer in 2008: GLOBOCAN 2008. Int J Cancer. 2010; 127:2893–2917.
3. Brooks NA, O'Donnell MA. Treatment options in non-muscle-invasive bladder cancer after BCG failure. Indian J Urol. 2015; 31:312–319.
4. Mani J, Vallo S, Rakel S, Antonietti P, Gessler F, Blaheta R, et al. Chemoresistance is associated with increased cytoprotective autophagy and diminished apoptosis in bladder cancer cells treated with the BH3 mimetic (-)-Gossypol (AT-101). BMC Cancer. 2015; 15:224.
5. Kaufman DS, Shipley WU, Feldman AS. Bladder cancer. Lancet. 2009; 374:239–249.
6. Meeks JJ, Bellmunt J, Bochner BH, Clarke NW, Daneshmand S, Galsky MD, et al. A systematic review of neoadjuvant and adjuvant chemotherapy for muscle-invasive bladder cancer. Eur Urol. 2012; 62:523–533.
7. Porter MP, Kerrigan MC, Donato BM, Ramsey SD. Patterns of use of systemic chemotherapy for Medicare beneficiaries with urothelial bladder cancer. Urol Oncol. 2011; 29:252–258.
8. Hanahan D, Weinberg RA. Hallmarks of cancer: the next generation. Cell. 2011; 144:646–674.
9. Klionsky DJ, Emr SD. Autophagy as a regulated pathway of cellular degradation. Science. 2000; 290:1717–1721.
10. Mizushima N, Levine B, Cuervo AM, Klionsky DJ. Autophagy fights disease through cellular self-digestion. Nature. 2008; 451:1069–1075.
11. Chen N, Karantza V. Autophagy as a therapeutic target in cancer. Cancer Biol Ther. 2011; 11:157–168.
12. Leone RD, Amaravadi RK. Autophagy: a targetable linchpin of cancer cell metabolism. Trends Endocrinol Metab. 2013; 24:209–217.
13. Farrow JM, Yang JC, Evans CP. Autophagy as a modulator and target in prostate cancer. Nat Rev Urol. 2014; 11:508–516.
14. Mukhopadhyay S, Sinha N, Das DN, Panda PK, Naik PP, Bhutia SK. Clinical relevance of autophagic therapy in cancer: Investigating the current trends, challenges, and future prospects. Crit Rev Clin Lab Sci. 2016; 53:228–252.
15. Rebecca VW, Amaravadi RK. Emerging strategies to effectively target autophagy in cancer. Oncogene. 2016; 35:1–11.
16. Nguyen HG, Yang JC, Kung HJ, Shi XB, Tilki D, Lara PN Jr, et al. Targeting autophagy overcomes Enzalutamide resistance in castration-resistant prostate cancer cells and improves therapeutic response in a xenograft model. Oncogene. 2014; 33:4521–4530.
17. van Rhijn BW, Burger M, Lotan Y, Solsona E, Stief CG, Sylvester RJ, et al. Recurrence and progression of disease in non-muscle-invasive bladder cancer: from epidemiology to treatment strategy. Eur Urol. 2009; 56:430–442.
18. Ojha R, Singh SK, Bhattacharyya S, Dhanda RS, Rakha A, Mandal AK, et al. Inhibition of grade dependent autophagy in urothelial carcinoma increases cell death under nutritional limiting condition and potentiates the cytotoxicity of chemotherapeutic agent. J Urol. 2014; 191:1889–1898.
19. Sivridis E, Koukourakis MI, Mendrinos SE, Touloupidis S, Giatromanolaki A. Patterns of autophagy in urothelial cell carcinomas: the significance of "stone-like" structures (SLS) in transurethral resection biopsies. Urol Oncol. 2013; 31:1254–1260.
20. Lin YC, Lin JF, Wen SI, Yang SC, Tsai TF, Chen HE, et al. Inhibition of High Basal Level of Autophagy Induces Apoptosis in Human Bladder Cancer Cells. J Urol. 2015; 10. 28. [Epub]. DOI: 10.1016/j.juro.2015.10.128.
21. Baspinar S, Bircan S, Yavuz G, Kapucuoglu N. Beclin 1 and bcl-2 expressions in bladder urothelial tumors and their association with clinicopathological parameters. Pathol Res Pract. 2013; 209:418–423.
22. Shen B, Tan M, Mu X, Qin Y, Zhang F, Liu Y, et al. Upregulated SMYD3 promotes bladder cancer progression by targeting BCLAF1 and activating autophagy. Tumour Biol. 2016; 37:7371–7381.
23. Gregg JR, Dahm P, Chang SS. Guideline-based management of non-muscle invasive bladder cancer. Indian J Urol. 2015; 31:320–326.
24. Porten SP, Leapman MS, Greene KL. Intravesical chemotherapy in non-muscle-invasive bladder cancer. Indian J Urol. 2015; 31:297–303.
25. Ojha R, Jha V, Singh SK. Gemcitabine and mitomycin induced autophagy regulates cancer stem cell pool in urothelial carcinoma cells. Biochim Biophys Acta. 2016; 1863:347–359.
26. Babjuk M, Burger M, Zigeuner R, Shariat SF, van Rhijn BW, Comperat E, et al. EAU guidelines on non-muscle-invasive urothelial carcinoma of the bladder: update 2013. Eur Urol. 2013; 64:639–653.
27. Hall MC, Chang SS, Dalbagni G, Pruthi RS, Seigne JD, Skinner EC, et al. Guideline for the management of nonmuscle invasive bladder cancer (stages Ta, T1, and Tis): 2007 update. J Urol. 2007; 178:2314–2330.
28. Buffen K, Oosting M, Quintin J, Ng A, Kleinnijenhuis J, Kumar V, et al. Autophagy controls BCG-induced trained immunity and the response to intravesical BCG therapy for bladder cancer. PLoS Pathog. 2014; 10:e1004485.
29. Steinberg G, Bahnson R, Brosman S, Middleton R, Wajsman Z, Wehle M. Efficacy and safety of valrubicin for the treatment of Bacillus Calmette-Guerin refractory carcinoma in situ of the bladder. The Valrubicin Study Group. J Urol. 2000; 163:761–767.
30. Askeland EJ, Newton MR, ODonnell MA, Luo Y. Bladder cancer immunotherapy: BCG and Beyond. Adv Urol. 2012; 2012:181987.
31. O'Donnell MA, Krohn J, DeWolf WC. Salvage intravesical therapy with interferon-alpha 2b plus low dose bacillus Calmette-Guerin is effective in patients with superficial bladder cancer in whom bacillus Calmette-Guerin alone previously failed. J Urol. 2001; 166:1300–1304.
32. Lam JS, Benson MC, O'Donnell MA, Sawczuk A, Gavazzi A, Wechsler MH, et al. Bacillus Calmete-Guérin plus interferon-alpha2B intravesical therapy maintains an extended treatment plan for superficial bladder cancer with minimal toxicity. Urol Oncol. 2003; 21:354–360.
33. Benedict WF, Tao Z, Kim CS, Zhang X, Zhou JH, Adam L, et al. Intravesical Ad-IFNalpha causes marked regression of human bladder cancer growing orthotopically in nude mice and overcomes resistance to IFN-alpha protein. Mol Ther. 2004; 10:525–532.
34. Zhang XQ, Dunner K Jr, Benedict WF. Autophagy is induced by adenoviral-mediated interferon alpha treatment in interferon resistant bladder cancer and normal urothelial cells as a cell death protective mechanism but not by the bystander factors produced. Cancer Gene Ther. 2010; 17:579–584.
35. Cockerill PA, Knoedler JJ, Frank I, Tarrell R, Karnes RJ. Intravesical gemcitabine in combination with mitomycin C as salvage treatment in recurrent non-muscle-invasive bladder cancer. BJU Int. 2016; 117:456–462.
36. Pan XW, Li L, Huang Y, Huang H, Xu DF, Gao Y, et al. Icaritin acts synergistically with epirubicin to suppress bladder cancer growth through inhibition of autophagy. Oncol Rep. 2016; 35:334–342.
37. Li K, Chen X, Liu C, Gu P, Li Z, Wu S, et al. Pirarubicin induces an autophagic cytoprotective response through suppression of the mammalian target of rapamycin signaling pathway in human bladder cancer cells. Biochem Biophys Res Commun. 2015; 460:380–385.
38. Arum CJ, Gederaas OA, Larsen EL, Randeberg LL, Hjelde A, Krokan HE, et al. Tissue responses to hexyl 5-aminolevulinate-induced photodynamic treatment in syngeneic orthotopic rat bladder cancer model: possible pathways of action. J Biomed Opt. 2011; 16:028001.
39. Knollman H, Godwin JL, Jain R, Wong YN, Plimack ER, Geynisman DM. Muscle-invasive urothelial bladder cancer: an update on systemic therapy. Ther Adv Urol. 2015; 7:312–330.
40. International Collaboration of Trialists. Medical Research Council Advanced Bladder Cancer Working Party (now the National Cancer Research Institute Bladder Cancer Clinical Studies Group). European Organisation for Research and Treatment of Cancer Genito-Urinary Tract Cancer Group. Australian Bladder Cancer Study Group. National Cancer Institute of Canada Clinical Trials Group. Finnbladder. . International phase III trial assessing neoadjuvant cisplatin, methotrexate, and vinblastine chemotherapy for muscle-invasive bladder cancer: long-term results of the BA06 30894 trial. J Clin Oncol. 2011; 29:2171–2177.
41. Chou R, Selph SS, Buckley DI, Gustafson KS, Griffin JC, Grusing SE, et al. Treatment of muscle-invasive bladder cancer: A systematic review. Cancer. 2016; 122:842–851.
42. Huang YT, Cheng CC, Lin TC, Chiu TH, Lai PC. Therapeutic potential of sepantronium bromide YM155 in gemcitabine-resistant human urothelial carcinoma cells. Oncol Rep. 2014; 31:771–780.
43. Li JR, Cheng CL, Yang WJ, Yang CR, Ou YC, Wu MJ, et al. FIP-gts potentiate autophagic cell death against cisplatin-resistant urothelial cancer cells. Anticancer Res. 2014; 34:2973–2983.
44. Li JR, Cheng CL, Yang CR, Ou YC, Wu MJ, Ko JL. Dual inhibitor of phosphoinositide 3-kinase/mammalian target of rapamycin NVP-BEZ235 effectively inhibits cisplatin-resistant urothelial cancer cell growth through autophagic flux. Toxicol Lett. 2013; 220:267–276.
45. Zhang DQ, Zhou CK, Jiang XW, Chen J, Shi BK. Increased expression of miR-222 is associated with poor prognosis in bladder cancer. World J Surg Oncol. 2014; 12:241.
46. Zeng LP, Hu ZM, Li K, Xia K. miR-222 attenuates cisplatin-induced cell death by targeting the PPP2R2A/Akt/mTOR Axis in bladder cancer cells. J Cell Mol Med. 2016; 20:559–567.
47. Pinto-Leite R, Arantes-Rodrigues R, Ferreira R, Palmeira C, Colaco A, Moreira da Silva V, et al. Temsirolimus improves cytotoxic efficacy of cisplatin and gemcitabine against urinary bladder cancer cell lines. Urol Oncol. 2014; 32:41.e11. 41.e22.
48. Mathieu R, Lucca I, Klatte T, Babjuk M, Shariat SF. Trimodal therapy for invasive bladder cancer: is it really equal to radical cystectomy? Curr Opin Urol. 2015; 25:476–482.
49. Shrivastava S, Mansure JJ, Almajed W, Cury F, Ferbeyre G, Popovic M, et al. The Role of HMGB1 in Radioresistance of Bladder Cancer. Mol Cancer Ther. 2016; 15:471–479.




 PDF
PDF Citation
Citation Print
Print


 XML Download
XML Download