Abstract
Schwannomas are benign slow-growing nerve sheath tumors, which can develop in any peripheral or central nerve that contains Schwann cells. Schwannomas located near the olfactory groove are extremely rare and radiological diagnosis can be difficult. Moreover, ancient schwannoma is an uncommon variant, and radiologic findings are rarely reported. Herein, we reported a surgically confirmed case of ancient schwannoma at the olfactory groove in a 44-year-old woman presenting with headache and visual disturbance. Brain magnetic resonance imaging (MRI) showed a solid and cystic extra-axial mass located in the subfrontal area mimicking an olfactory groove meningioma. Histopathologic diagnosis of ancient schwannoma was confirmed by immunohistochemical staining for S100, CD56, vimentin, and other markers. Furthermore, we described the clinical manifestations, MRI characteristics, and histopathologic findings of the case, and presented a review of related literature.
Schwannomas are benign slow-growing nerve sheath tumors that can develop in any peripheral or central nerve that contains Schwann cells. Most intracranial schwannomas arise from the vestibular, trigeminal, lower cranial, or facial nerves, in descending order. Theoretically, schwannomas cannot develop from the optic or olfactory nerves because they lack a Schwann cell layer (1). The origin of schwannomas located at the olfactory groove remains uncertain, but, they are thought to arise from branches of the first division of the trigeminal nerve or from the fila olfactoria (2). Solitary olfactory groove schwannomas are extremely rare and often preoperatively misdiagnosed as meningioma.
In addition, ancient schwannoma is a rare variant that usually has a deep location with prolonged duration (3). To the best of our knowledge, there is no report of the radiologic findings of ancient schwannoma located at the olfactory groove.
A 44-year-old woman visited our hospital with abnormal brain magnetic resonance imaging (MRI) findings obtained at a local clinic 3 days prior. She reported headache of 1 month duration and visual disturbance (blurred vision and diplopia) for 1 week before admission. On physical examination, she demonstrated diplopia in straight gaze on the left side. However, she denied olfactory dysfunction. She had a medical history of breast cancer with complete remission 14 years prior. She had no signs or family history of neurofibromatosis.
MRI revealed a large, well-demarcated, lobulated, heterogeneously enhanced, extra-axial mass with cystic areas in the left subfrontal area (Fig. 1A-C). Solid enhancement in the anterior portion and a non-enhanced cystic area in the posterior portion were noted. In addition, some peritumoral edema was seen. The solid enhanced portion showed diffusion restriction on diffusion MRI (Fig. 1D) and increased relative cerebral blood volume (rCBV) on perfusion MRI (Fig. 1E). However, the non-enhanced cystic portion did not show diffusion restriction on diffusion MRI or increased rCBV on perfusion MRI. MR spectroscopy showed increased choline/creatine peak and decreased N-acetylaspartate peak, though interpretation was limited due to serious baseline distortion.
Tumor excision was performed via bifrontal craniotomy. Intraoperatively, the tumor was located at the left olfactory groove, attached to the anterior part of the cribriform plate, and adherent to the dura. There were no procedure-related complications, and the tumor nearly disappeared on pre-discharge follow-up computed tomography. The patient was discharged home with partial improvement of symptoms.
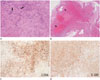
Histologic examination of the resected tissue revealed spindle-shaped cells with elongated nuclei and fibrillary cytoplasm (Antoni A pattern) (Fig. 2A), along with less cellular, loosely textured tumor areas (Antoni B). Extensive vascular hyalinization was seen (Fig. 2B), and immunohistochemical staining for CD56 (Fig. 2C), vimentin, and S100 (Fig. 2D) was positive. The pathologic diagnosis was confirmed as ancient schwannoma.
Intracranial schwannomas account for approximately 6% to 8% of all intracranial tumors (4). Schwannomas usually originate from the peripheral nerve sheath and occur less frequently in the central nervous system. Most intracranial schwannomas arise from the vestibulocochlear nerve, followed by the trigeminal nerve. Schwannomas with prominent degenerative change are referred to as ancient schwannomas, a very rare variant (3), which are characterized by diffuse hypocellular areas, relative loss of Antoni type A tissue, hyaline accumulation, calcification, cystic necrosis, hemorrhage, and fatty degeneration (5). This type of tumor may be misdiagnosed as malignancy due to its cellular degenerative changes. To date, there are only 36 cases of schwannoma in the anterior cranial fossa reported in the literature (1246789); moreover, there are no reports on the radiologic findings of ancient schwannoma.
Choi et al. (4) suggested that anterior cranial fossa schwannomas present different characteristics than lesions at other locations, such as earlier onset, male predominance, and a dominant solid tumor portion. Santhosh et al. (7) reported that schwannomas show multiple small hypointensities within the tumor on T2-weighted gradient-echo MRI sequence, which are presumed to be caused by microbleeds. In a literature review, Adachi et al. (6) reported that subfrontal schwannomas were solid and cystic and showed heterogeneous contrast enhancement. Luo et al. (10) suggested that extensive cystic degeneration is a characteristic finding of intracranial schwannoma not arising from the stems of the cranial nerves. Our patient showed similar findings, including an enhanced tumor with a cystic portion in the left subfrontal area, with microbleeds in the solid enhanced portion on T2-weighted gradient-echo MRI sequence. According to previous reports, extramural/arachnoid cysts may be caused by mechanical trapping of cerebrospinal fluid or by leakage of hemorrhagic material from the tumor, causing adhesion and secondary arachnoid cyst formation (9).
A previous report (1) suggested that the term "olfactory sch-wannoma" is incorrect because the olfactory bulb has no Sch-wann cell layer and is probably not the nerve of origin. Developmental and non-developmental hypotheses are suggested for the possible origin of olfactory schwannomas (8). According to developmental theories, mesenchymal pial cells are transferred to ectodermal Schwann cells, or neural crest cells migrate within the substance of the central nervous system (2). On the other hand, non-developmental theories suggest that schwannomas originate from the perivascular nerve plexus and meningeal branches of the trigeminal and anterior ethmoidal nerves, which innervate the anterior cranial fossa where Schwann cells are normally present (28).
Meningioma, esthesioneuroblastoma, metastasis, and others should be included in the differential diagnosis of extra-axial anterior cranial fossa neoplasm. Olfactory groove meningioma and schwannoma share similar radiologic features, including extra-axial location, calcification, enhancement pattern, and peritumoral edema. In our patient, the extra-axial mass was initially diagnosed radiologically as meningioma with peritumoral cyst based on its enhancement pattern and suspected dural tail sign on sagittal images. However, no definite bony erosion or sclerotic change of the cribriform plate was observed. Esthesioneuroblastoma was excluded from the differential diagnosis because it usually presents marked bony destruction. Metastasis was also excluded because of the unusual location, absence of extensive peritumoral edema, and the solitary mass.
Treatment options for schwannoma include surgical excision, observation, and radiotherapy, including Gamma knife surgery. However, complete tumor removal is the mainstay of treatment in order to prevent tumor recurrence. Moreover, patient prognosis after complete tumor resection is excellent.
In conclusion, schwannomas located at the olfactory groove are extremely rare and can be difficult to diagnose correctly before surgery. However, schwannoma should be considered in the differential diagnosis in cases of anterior cranial fossa neoplasm.
Figures and Tables
Fig. 1
A 44-year-old woman with headache and visual disturbance.
A, B. Contrast-enhanced T1-weighted axial (A) and sagittal (B) magnetic resonance (MR) images, showing a heterogeneously enhanced extra-axial mass with cystic areas in the left subfrontal area. Solid enhancement (arrow) in the anterior portion and a non-enhanced cystic area (arrowhead) in the posterior portion are noted. The lesion shows a clear margin with the surrounding tissue.
C. T2-weighted axial MR image showing a solid mass (arrow) surrounded by high signal intensity, implying a cystic component (arrowhead).
D. Diffusion MR image showing no definite diffusion restriction in the solid portion.
E. Perfusion MR image showing increased relative cerebral blood volume in the solid portion (arrow) and no definite change in the cystic portion.
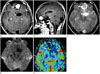
Fig. 2
Histologic examination, showing relatively compact areas of spindle cells (Antoni A pattern) (A), Verocay bodies are formed by palisade Schwann cells (arrows) (magnification × 40) (B). Extensive vascular hyalinization (arrowheads), one of the degenerative changes of ancient schwannoma is seen (magnification × 10). Diffuse staining for CD56 (C) and S100 (D) in cell nuclei and cytoplasm, which is typically more prominent in hypercellular areas (Antoni A) than in hypocellular areas (Antoni B), is noted (magnification × 40).
References
1. Park JH, Kim TY, Park JT, Kim JM. Olfactory groove schwannoma. J Korean Neurosurg Soc. 2006; 39:156–158.
2. Urich H, Tien RD. Tumors of the cranial, spinal and peripheral nerve sheaths. In : Bigner DD, McLendon RE, Bruner JM, Russell DS, editors. Russell and Rubinstein's pathology of tumours of the nervous system. London, New York: Arnold, Oxford University Press;1988. p. 141–193.
3. Lee YS, Kim JO, Park SE. Ancient schwannoma of the thigh mimicking a malignant tumour: a report of two cases, with emphasis on MRI findings. Br J Radiol. 2010; 83:e154–e157.
4. Choi YS, Sung KS, Song YJ, Kim HD. Olfactory schwannoma-case report. J Korean Neurosurg Soc. 2009; 45:103–106.
5. Isobe K, Shimizu T, Akahane T, Kato H. Imaging of ancient schwannoma. AJR Am J Roentgenol. 2004; 183:331–336.
6. Adachi K, Yoshida K, Miwa T, Ikeda E, Kawase T. Olfactory schwannoma. Acta Neurochir (Wien). 2007; 149:605–610. discussion 610-611.
7. Santhosh K, Kesavadas C, Radhakrishnan VV, Thomas B, Kapilamoorthy TR, Gupta AK. Usefulness of T2*-weighted MR sequence for the diagnosis of subfrontal schwannoma. J Neuroradiol. 2007; 34:330–333.
8. Timothy J, Chakrabarty A, Rice A, Marks P. Olfactory groove schwannoma revisited. Acta Neurochir (Wien). 1999; 141:671–672.
9. Zagardo MT, Castellani RJ, Rees JH, Rothman MI, Zoarski GH. Radiologic and pathologic findings of intracerebral schwannoma. AJNR Am J Neuroradiol. 1998; 19:1290–1293.
10. Luo B, Sun G, Zhang B, Liang K, Wen J, Fang K. Neuroradiological findings of intracranial schwannomas not arising from the stems of cranial nerves. Br J Radiol. 2004; 77:1016–1021.




 PDF
PDF ePub
ePub Citation
Citation Print
Print


 XML Download
XML Download