Abstract
Purpose
The purpose of this study is to analyze the computed tomography (CT) features of follicular thyroid carcinoma (FTC) as compared to those of papillary thyroid carcinoma (PTC) to identify the characteristic imaging features of FTC.
Materials and Methods
The following CT features were analyzed to compare FTCs (n = 17) and PTCs (n = 27): size, shape, margin, internal composition, calcification, enhancement homogeniety, extrathyroidal extension, lymph node (LN) metastasis and the density and degree of enhancement (qualitatively and quantitatively).
Results
There were no significant differences between the patients with FTCs and those with PTCs with respect to age and gender, and the internal composition, calcification and enhancement homogeneity of the tumor. However, the FTCs tend to show a larger size (32 mm vs. 15 mm; respectively, p < 0.001), a round to oval shape (52.9% vs. 14.8% respectively, p = 0.001), a well-defined smooth margin (58.8% vs. 14.8% respectively, p = 0.009), less extrathyroidal extension (11.8% vs. 51.9% respectively, p = 0.007) and less LN metastasis (0% vs. 29.6% respectively, p = 0.016) compared to that of the PTCs. Furthermore, the FTCs showed significantly stronger enhancement in the early phase (146.4±42.4 vs. 98.5±38.2 respectively, p < 0.001) and a greater decrement of the late enhancement (-44.6±25.2 vs. -18.7±27.9 respectively, p = 0.003) compared to that of the PTCs.
Follicular thyroid carcinomas (FTCs) are relatively uncommon types of thyroid malignancies (1). Patients with FTCs occasionally present at a more advanced stage of disease with a higher incidence of distant metastases as compared to that of patients with papillary thyroid carcinomas (23). Because of its propensity for vascular invasion, FTCs sometimes present with bone, lung or soft tissue metastases at the time of the initial diagnosis (2). According to the data from the Surveillance, Epidemiology and National Cancer Institute, the incidence rate of FTCs has remained stable, but the incidence of FTCs < 2 cm in size has increased at the same rate as that for FTCs > 2 cm in size (4). This statistic may be due to the wide use of ultrasonography (US) and use of US-guided fine needle aspiration (FNA), which has led to more surgical procedures, and this in turn confirms more of the smaller FTCs. Also, cytologic assessment of FNA biopsy specimens has been shown to be an ineffective method for diagnosing FTCs, and this often leads to having to using multiple frozen sections for making the pathologic diagnosis (2).
Jeh et al. (5) previously reported on the US findings of malignant thyroid tumor, and they suggested that FTC could have imaging features that are distinct from those of PTC. In our daily practice, we had noticed that FTC frequently showed a round to oval shape with prominent enhancement patterns on neck CT. Thus, we analyzed CT findings of FTCs as compared to the CT findings of PTCs in order to identify the imaging features that are characteristic of FTCs, based on neck CT scans.
We retrospectively reviewed the medical records of all the patients with thyroid malignancies and who were seen at our hospital between January 2007 and December 2009. Twenty one patients with FTCs and 590 patients with PTCs were consecutively diagnosed by the surgical pathology and they underwent pre-operative head and neck CT at our institution. Every tenth patient among the 590 patients with PTCs was selected for a comparison with the patients with FTCs. Twenty one patients with FTCs and 59 patients with PTCs were initially included for this study. However, thirty-six of these patients were excluded during the image analysis by the following exclusion criteria: the maximum diameter of the tumor less than 1 cm (n=17), there was underlying diffuse thyroid disease (n=12), there was the possibility of mismatching multiple nodules that were not correlated with the surgical pathology result (n=4), there were overlapping CT artifacts (n=1) and there was dense calcified nodule without a solid portion (n=2). In such cases, detection of nodule and the evaluation of shape, margin, enhancement homogeneity and attenuation relative to the parenchyma were nearly impossible. Underlying diffuse thyroid disease was determined by the surgical pathology or imaging features such as prominent diffuse enlargement, an irregular contour and parenchymal enhancement distinct from that of the normal thyroid parenchyma. Finally, the neck CT scans of 17 patients (6 males and 11 females, mean age: 48.2 ± 15.9 years) with FTCs and 27 patients (3 males and 24 females, mean age: 44.7 ± 12.9 years) with PTCs were analyzed in the current study. The seventeen FTCs were composed of 11 minimally invasive papillary carcinomas, 2 widely invasive papillary carcinomas, 3 Hurthle cell carcinomas and 1 follicular variant of papillary carcinomas.
All the CT scans were performed using a 40-detector row CT scanner (Philips Medical System, Best, Netherlands) with the following parameters: a 5-mm section thickness, a pitch of 1.5, 4 × 1.5-mm collimation, 120 kV and 250 mAs. The scanning range covered the maxillary sinus to the tracheal bifurcation. For the contrast enhancement study, 100 ml of non-ionic contrast medium (Omipaque 300, 300 mgI/mL; Schering, Osaka, Japan) was intravenously administered at a rate of 2 ml/s using a power injector. After the pre-contrast scan, the early- and delay-phase CE images were scanned with a delay of 30 and 50 seconds, respectively. In addition to the axial images, the coronal multiplanar reconstruction (MPR) images were obtained with a 3 mm slice thickness.
Two radiologists conducted a retrospective consensus review of all the CT images of the 17 patients with FTCs and the 27 patients with PTCs on a picture archiving and communication system workstation (Infinitt Technology, Seoul, Korea). The reviewers were 'blind' to the final surgical pathology such as FTCs or PTCs, and all the CT images for malignant thyroid nodules were presented at random. The following CT features were analyzed: size (maximum diameter), shape (round-to-oval vs. taller than wide vs. geographic, internal composition (predominantly solid vs. predominantly cystic), margin (well-defined smooth vs. irregular or ill-defined), calcifications (absence or presence), enhancement homogeneity (homogeneous vs. heterogeneous), extrathyroidal extension (absence or presence), lymph node (LN) metastasis (absence or presence), density and the degree of enhancement (the qualitative analysis by visual assessment and the quantitative analysis by absolute assessment). For extrathyroidal extension, an ill-defined margin with obliteration of the adjacent fat plane was thought to be a suspicious finding. As diagnostic criteria for LN metastasis, we adopted the morphologic and size criteria that were used in the report by Son et al. (6). Specifically, at least one of the following criteria was required: strong nodal enhancement without hilar vessel enhancement, heterogeneous enhancement, calcifications, cystic changes and a short transverse diameter > 5 mm for the central neck level and 8 mm for the lateral neck level. Finally, the imaging findings that were suspicious for extrathyroidal extension and LN metastasis were regarded as positive only in the cases that had concordance with the surgical pathology.
For qualitative analysis, the thyroid nodules were visually assessed and classified into one of three groups (hypodense, isodense and hyperdense). The assessment was based on the relative attenuation of the nodule compared with the adjacent parenchyma on each phase of the CT; the patients with underlying diffuse thyroid disease that showed a variety of parenchymal attenuation were excluded from this study. The radiologists selected a solid area showing the highest attenuation during any phase and they measured the average HUs using a circle-shaped region of interest (ROI) with a diameter more than 5 mm at the same level of each phase. The degrees of early and late enhancement were calculated as the HU on the early phase scan - the HU on the unenhanced scan, and the HU on the delayed phase scan - the HU on the early phase scan.
The χ2 test or Fisher's exact test was used to compare the following parameters for the FTCs and PTCs: gender, the character of the shape, margin, the internal composition and enhancement homogeneity, the absence or presence of calcification, extrathyroidal extension and LN metastasis, and the nodule density by visual assessment. Student's t-test and the Mann-Whitney U test were used for assessing age, size and nodule density and the degree of enhancement by the absolute assessment. The normal distribution of the data in each group was assessed with the Kolmogorov-Smirnov test before the Student's t-test. A two-tailed p value < 0.05 was considered to indicate a significant difference. The data analyses were performed with commercially available software (SPSS for Windows, version 16.0; SPSS, Inc., Chicago, IL, USA).
The demographics and morphologic CT findings for the FTCs (n=17) and PTCs (n=27) are summarized in Table 1. There was no significant predilection between the FTCs and PTCs according to age and gender, and no significant difference existed in the internal composition, calcification and enhancement homogeneity (p > 0.05). However, there were significant differences between the FTCs and PTCs with respect to size, shape, margin, extrathyroidal extension and LN metastasis (p < 0.05). The FTCs tended to be larger than the PTCs (33.7 ± 14.9 mm vs. 17.2 ± 6.3 mm, respectively; p < 0.001) and they more frequently had a round to oval shape (52.9% vs. 14.8%, respectively; p = 0.001) and a well-defined smooth margin (58.8% vs. 14.8%, respectively; p = 0.009) (Fig. 1). In addition, the possibility of extrathyroidal extension (11.8% vs. 51.9%, respectively; p = 0.007) and LN metastasis (0% vs. 29.6%, respectively; p = 0.016) was significantly lower in the FTCs than that in the PTCs.
On the unenhanced images, all the nodules were hypodense relative to the thyroid parenchyma. At the early phase, 7 FTCs (41.2%) were hyperdense, 8 FTCs (47.1%) were isodense and 2 FTC (11.8%) was hypodense. In contrast, most of the PTCs (n=21 [77.8%]) were hypodense, although 5 PTCs (18.5%) were isodense. On the delayed phase, 5 FTCs (29.4%) were hyperdense, 4 FTCs (23.5%) were isodense and 8 FTCs (47.1%) were hypodense. For the PTCs, 25 (92.6%) were hypodense and 2 were isodense. Also, most of the hyperdense nodules on any phase (87.5%) were observed in the FTCs and the portion of nodules that showed more than isodense enhancement on both phases (Fig. 1) was significantly higher for the FTCs than that for the PTCs (52.9% vs. 0%, respectively; p < 0.001). In contrast, the portion of nodules that showed less than hypodense enhancement on both phases (Fig. 2) was significantly lower for the FTCs than that for the PTCs (11.8% vs. 74.1%, respectively; p < 0.001) (Table 2) .
On the unenhanced images, there was no significant difference in the average HUs between the FTCs and PTCs (p > 0.05). On the early and delayed phases, the average HUs of the FTCs was significantly higher than that of the PTCs (213.3 ± 45.1 vs. 169.8 ± 30.9, respectively; p < 0.001 on the early phase and 162.7 ± 31.3 vs. 136.4 ± 28.9, respectively; p = 0.007 on the delayed phase). A significant difference was also noted in the degree of early and late enhancement; the FTCs showed significantly stronger enhancement on the early phase (146.4±42.4 vs. 98.5 ± 38.2, respectively; p < 0.001) and a significantly greater decrement on the late enhancement (−44.6 ± 25.2 vs. −18.7 ± 27.9, respectively; p < 0.003) as compared to that of the PTCs (Table 2).
Thyroid follicular neoplasm is usually diagnosed following FNA biopsies of a dominant thyroid nodule(8), but cytologic diagnoses are often challenging to make for FTCs because of the overlapping cytologic features (9). The risk of carcinoma is approximately 20% on the basis of cytologic diagnoses (8) and many studies have been conducted to differentiate malignancies using imaging modalities (10111213). The US features alone cannot distinguish benign from malignant nodules because not only the cytologic features overlap, but also the morphologies are also extremely similar. Based on the recent epidemiologic data of FTCs, their size has decreased compared to that of the past (4), which implies that more surgical procedures are being performed to diagnose the approximate 20% of the malignancies among all the follicular tumors. The recent high resolution imaging studies have used US in the evaluation of thyroid follicular nodules (1415). But studies focusing on the CT findings of thyroid nodules have rarely been performed. To the best of our knowledge, some reports have suggested that the CT features, such as calcifications, a taller than wide shape and a mean attenuation >130 HU in a dominant nodule is suggestive of a malignancy (16), while others have shown that there are no CT features that can distinguish benign from malignant lesions (17). Yet we focused our study on the differentiation of CT features between FTCs and PTCs because the clinical manifestations and the clinical courses or outcomes are somewhat different (318). We expected to find some unique CT features and clinical characteristics of FTCs as compared to those of PTCs.
The morphologic CT findings, such as a round to oval shape and a well-defined smooth margin were significantly more frequent in FTCs compared to that of PTCs, but extrathyroidal extension and LN metastasis were significantly less frequent in FTCs compared to that of PTCs. The result that an oval or round shape and a well-defined smooth margin were more frequent in FTCs than that in PTCs is supported by the opinion of Jeh et al. (5), and it is consistent with the study by Jung et al. (19), who reported that the relatively high frequency of round- or oval-shaped FTCs made the differential diagnosis for malignancy difficult. When considering the pathological features that PTC is an un-encapsulated tumor with papillary and follicular structures and the tumor cells are characterized by nuclear atypia, but FTC is encapsulated and differentiated from follicular adenomas by invasion of the capsule and vessels, such morphologic features of FTCs were thought to be closely associated with the pathologic feature of encapsulation. Our study also showed that FTCs make up the majority of nodules with hyperdense enhancement on any phase (87.5%) and all the nodules with enhancement more than isodense on all phases. Considering that most of the nodules with hypodense enhancement on all phases were PTCs (90.9%), such findings of FTCs were worthy of notice as the most prominent CT features that were distinct from those of PTCs (Fig. 1). Especially, FTCs showed somewhat characteristic features of strong enhancement on the early phase and a prominent decrement in enhancement on the delay phase as compared to that of PTCs. Several studies using color Doppler US to predict malignancy in follicular neoplasm (10142021) were somewhat helpful in predicting or describing the tumor character, but the studies were not conclusive. On the contrary, the PTCs had a tendency to display as irregular or ill-defined, taller than wide hypodense nodules on unenhanced CT and the PTCs showed less enhancement relative to the thyroid parenchyma on the early and delayed phases (Fig. 2).
This study showed that FTCs showed a significantly larger size than did the PTCs and this finding may support the results of several previous studies that reported tumor size as a risk factor for FTCs (1522). This finding may implicate that malignancy may be derived from progressive dedifferentiation from a pre-existing follicular adenoma and so this reflects the stepwise transformation from adenoma to invasive carcinoma (232425). We think that follicular carcinomas resemble benign nodule for the shape and margins, and so biopsies can be excluded until the tumor reaches a certain size: thus, when the nodule is diagnosed its size is considerably larger compared to that of PTC. According to the AACE/AME Task Force (26), a consensus has not been reached regarding the clinical significance of tumor size, which can alter the indication for performing a FNA biopsy on benign nodules. There is little data to predict benign nodules being 100% benign other than as confirmed by FNA biopsies, so performing FNA biopsies on nodules > 1 cm has substantially increased, and as a consequence this may lead to more frequent excisional biopsies. We believe that nodules that are incidentally detected and that have the cytological results of follicular neoplasm should not undergo surgical excision unless there is strong evidence of malignancy on the imaging studies, such as invasion to adjacent anatomic structures, cervical lymph node metastasis and/or other distant metastasis.
This is a retrospective imaging analysis and so it has inherent limitations. First, we did not compare follicular adenomas and follicular carcinomas in order to characterize the significant image features. There is a report showing that no CT feature can reliably distinguish benign from malignant lesions in the thyroid gland (17). Second, the sample size of the subjects was too small to achieve statistical significance due to the low prevalence of FTCs, and the pathologic subtypes of follicular carcinomas such as minimally invasive or widely invasive were not separately categorized. Third, we excluded small nodules < 1 cm in size because there is a limitation in characterizing the imaging features on head and neck CTs due to a variety of artifacts, summation effects and beam-hardening artifacts.
In conclusion, this study demonstrated that FTCs have somewhat characteristic CT features, such as oval or round shapes, a well-defined smooth margin, strong enhancement and a definite decrement on the delay phase as compared to that of the PTCs.
Figures and Tables
Fig. 1
Follicular thyroid carcinoma proven by surgical resection
On the unenhanced CT (A), a well-defined smooth round to oval hypodense mass (*) is seen in the left lobe of the thyroid gland. On the early (B) and delayed phases (C), the mass (arrowed) shows strong enhancement relative to the thyroid parenchyma.

Fig. 2
Papillary thyroid carcinoma proven by surgical resection
On the unenhanced CT (A), an ill-defined or irregular taller than wide hypodense mass (*) is seen in the left lobe of the thyroid gland. In the early (B) and delayed phases (C), the mass (arrowhead) shows less enhancement relative to the thyroid parenchyma. On the coronary MPR image (D), the mass shows definite extrathyroidal extension (arrows).
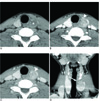
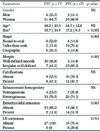
Table 1
Differences in the Morphologic CT Findings Between the Follicular and Papillary Thyroid Cancers
References
1. Widder S, Guggisberg K, Khalil M, Pasieka JL. A pathologic re-review of follicular thyroid neoplasms: the impact of changing the threshold for the diagnosis of the follicular variant of papillary thyroid carcinoma. Surgery. 2008; 144:80–85.
2. Lin JD, Chao TC. Follicular thyroid carcinoma: from diagnosis to treatment. Endocr J. 2006; 53:441–448.
3. Lo CY, Chan WF, Lam KY, Wan KY. Follicular thyroid carcinoma: the role of histology and staging systems in predicting survival. Ann Surg. 2005; 242:708–715.
4. Albores-Saavedra J, Henson DE, Glazer E, Schwartz AM. Changing patterns in the incidence and survival of thyroid cancer with follicular phenotype--papillary, follicular, and anaplastic: a morphological and epidemiological study. Endocr Pathol. 2007; 18:1–7.
5. Jeh SK, Jung SL, Kim BS, Lee YS. Evaluating the degree of conformity of papillary carcinoma and follicular carcinoma to the reported ultrasonographic findings of malignant thyroid tumor. Korean J Radiol. 2007; 8:192–197.
6. Son KR, Na DG, Chang KH. Diagnostic value of CT for the detection of cervical lymph node metastases in papillary thyroid carcinoma. J Korean Soc Radiol. 2009; 60:383–389.
7. AACE/AME Task Force on Thyroid Nodules. American Association of Clinical Endocrinologists and Associazione Medici Endocrinologi medical guidelines for clinical practice for the diagnosis and management of thyroid nodules. Endocr Pract. 2006; 12:63–102.
8. Carling T, Udelsman R. Follicular neoplasms of the thyroid: what to recommend. Thyroid. 2005; 15:583–587.
9. Ponikiewska D, Szczesniak-Klusek B, Stobiecka E, Jaworska M, Lange D. Oxyphilic and follicular thyroid tumors: the correlation between the cytopathologic diagnosis and the histopathologic examination. Endokrynol Pol. 2006; 57:Suppl A. 7–11.
10. Frates MC, Benson CB, Doubilet PM, Cibas ES, Marqusee E. Can color Doppler sonography aid in the prediction of malignancy of thyroid nodules. J Ultrasound Med. 2003; 22:127–131. quiz 132-124.
11. Lee B, Cook G, John L, Harrington K, Nutting C. Follicular thyroid carcinoma metastasis to the esophagus detected by 18FDG PET/CT. Thyroid. 2008; 18:267–271.
12. Talbot JN, Montravers F, Younsi N, Zanotti-Fregonara P, Grahek D, Kerrou K, et al. PET in thyroid cancers. Presse Med. 2006; 35:1377–1385.
13. Smith RB, Robinson RA, Hoffman HT, Graham MM. Preoperative FDG-PET imaging to assess the malignant potential of follicular neoplasms of the thyroid. Otolaryngol Head Neck Surg. 2008; 138:101–106.
14. Choi YJ, Yun JS, Kim DH. Clinical and ultrasound features of cytology diagnosed follicular neoplasm. Endocr J. 2009; 56:383–389.
15. Sillery JC, Reading CC, Charboneau JW, Henrichsen TL, Hay ID, Mandrekar JN. Thyroid follicular carcinoma: sonographic features of 50 cases. AJR Am J Roentgenol. 2010; 194:44–54.
16. Yoon DY, Chang SK, Choi CS, Yun EJ, Seo YL, Nam ES, et al. The prevalence and significance of incidental thyroid nodules identified on computed tomography. J Comput Assist Tomogr. 2008; 32:810–815.
17. Shetty SK, Maher MM, Hahn PF, Halpern EF, Aquino SL. Significance of incidental thyroid lesions detected on CT: correlation among CT, sonography, and pathology. AJR Am J Roentgenol. 2006; 187:1349–1356.
18. Lang BH, Lo CY, Chan WF, Lam KY, Wan KY. Staging systems for follicular thyroid carcinoma: application to 171 consecutive patients treated in a tertiary referral centre. Endocr Relat Cancer. 2007; 14:29–42.
19. Jung JH, Hwang GH, Park HY, Lee YH. Role of Ultrasonography in differential diagnosis of thyroid nodules. J Korean Surg Soc. 2006; 70:349–356.
20. Fukunari N, Nagahama M, Sugino K, Mimura T, Ito K. Clinical evaluation of color Doppler imaging for the differential diagnosis of thyroid follicular lesions. World J Surg. 2004; 28:1261–1265.
21. Miyakawa M, Onoda N, Etoh M, Fukuda I, Takano K, Okamoto T, et al. Diagnosis of thyroid follicular carcinoma by the vascular pattern and velocimetric parameters using high resolution pulsed and power Doppler ultrasonography. Endocr J. 2005; 52:207–212.
22. Paramo JC, Mesko T. Age, tumor size, and in-office ultrasonography are predictive parameters of malignancy in follicular neoplasms of the thyroid. Endocr Pract. 2008; 14:447–451.
23. Kondo T, Ezzat S, Asa SL. Pathogenetic mechanisms in thyroid follicular-cell neoplasia. Nat Rev Cancer. 2006; 6:292–306.
24. Oertel YC. Follicular lesions of the thyroid gland: from hyperplasia to neoplasia. Endocr Pract. 2008; 14:251–252.
25. Sillery JC, Reading CC, Charboneau JW, Henrichsen TL, Hay ID, Mandrekar JN. Thyroid follicular carcinoma: sonographic features of 50 cases. AJR Am J Roentgenol. 2010; 194:44–54.
26. Gharib H, Papini E, Valcavi R, Baskin HJ, Crescenzi A, Dottorini ME, et al. American Association of Clinical Endocrinologists and Associazione Medici Endocrinologi medical guidelines for clinical practice for the diagnosis and management of thyroid nodules. Endocr Pract. 2006; 12:63–102.




 PDF
PDF ePub
ePub Citation
Citation Print
Print



 XML Download
XML Download