Abstract
Four proto-oncogenes commonly associated with well-differentiated thyroid carcinoma, rearranged during transfection (RET)/papillary thyroid cancer, BRAF, RAS, and PAX8/peroxisome proliferator activated receptor-γ, may carry diagnostic and prognostic significance. These oncogenes can be used to improve the diagnosis and management of well-differentiated thyroid carcinoma. Limited therapeutic options are available for patients with metastatic well-differentiated thyroid cancer, necessitating the development of novel therapies. Vascular endothelial growth factor (VEGF)- and RET-directed therapies such as sorafenib, motesanib, and sunitinib have been shown to be the most effective at inducing clinical responses and stabilizing the disease process. Further clinical trials of these therapeutic agents may soon change the management of thyroid cancer.
Remarkable advances have occurred in recent years in understanding the molecular biology of thyroid cancer. Various somatic mutations associated with the development and progression of thyroid carcinomas have been discovered [1,2,3,4]. Our rapidly expanding knowledge of the molecular genetics of thyroid cancer has shifted into clinical practice, offering better diagnostic accuracy and enabling the development of novel compounds for the treatment of advanced metastatic thyroid carcinoma [5,6,7,8]. As the most common and challenging problem in thyroid carcinoma is related to the diagnosis and prognostication of well-differentiated thyroid cancer (WDTC), this review will focus on the genetic abnormalities found in WDTC and their diagnostic and prognostic use, as well as on therapies targeted to specific mutated sites and kinase pathways.
The rearranged during transfection (RET) proto-oncogene is located on chromosome 10q11-12 and encodes a transmembrane receptor with a tyrosine kinase domain. Because it was first found only in papillary thyroid cancer (PTC), it is called RET/PTC [9]. Although more than ten rearrangements have been identified, RET/PTC1 and RET/PTC3 account for most of the rearrangements found in PTC [10]. The RET/PTC chimeric oncogenic protein leads to the activation of several downstream pathways, including mitogen-activated protein kinase (MAPK), extracellular signal-related kinase (ERK), and phosphatidylinositol 3-kinase (PI3K). The prevalence of RET/PTC rearrangements in sporadic papillary carcinoma is approximately 15% to 40% [11]. The RET/PTC rearrangement has also been detected in the follicular variant of PTC (FVPTC) [9]. Clinically, RET/PTC variants are often found in radiation-associated PTC. Following the Chernobyl nuclear plant explosion in 1986, there was an increase in pediatric PTC and an increase in the incidence of an aggressive solid variant of PTC that contained the RET/PTC3 rearrangement. A form of PTC with a later onset, with a more classical phenotype and clinical course, emerged in the Chernobyl survivors and contained RET/PTC1 [12].
The Rat sarcoma (RAS) oncogene families regulate two important signaling pathways in thyroid cancer, the MAP kinase cascade (Ras-Raf-MEK-ERK) and the PI3K/Akt signaling pathway. Mutations of H-RAS codon 61 and N-RAS codon 61 are the most common RAS mutations associated with thyroid cancer [13]. RAS mutations occur in both benign tumors and thyroid cancers (both PTC and follicular thyroid cancer [FTC]), with variable frequency in anaplastic thyroid cancers. PTC with RAS mutations often display a follicular architecture and are called FVPTC. PTCs carrying a RAS mutation tend to exhibit a lower rate of lymph node metastasis and are more likely to be encapsulated [14].
The MAP kinase pathway mediates cellular responses to growth signals [4]. The MAPK pathway consists of a three-tiered protein kinase cascade: an extracellular signal (growth factor) interacts with a receptor tyrosine kinase, which then stimulates the activity of a MAPK kinase kinase (a MAPKKK, e.g., Raf) via the G-protein Ras, which activates a MAPKK (e.g., MEK), which in turn activates a MAPK (e.g., ERK). ERK is able to phosphorylate a number of proteins that regulate cytoskeletal organization, metabolism, chromatin remodeling, and numerous transcription factors (Fig. 1). There are three kinds of Raf proteins: A-Raf, B-Raf, and C-Raf (or Raf-1). Activation of B-Raf occurs after binding to Ras alone, whereas for activation of A-Raf and C-Raf, additional signals are required [15]. B-Raf is therefore the most potent activator of downstream effector MEK kinases in the Raf-Raf-MEK-ERK pathway. Mutations in the MAP kinase pathway are found in approximately 30% of all cancers, while around 8% of cancers develop due to BRAF mutations [16,17].
BRAF mutations were first discovered in thyroid cancer in 2003 [1]. A missense mutation in exon 15, which occurs due to the substitution of the amino acid valine for glutamic acid at residue position 600 [16], is present in 36% to 69% of PTC cases and is the most frequent genetic change in PTC [18,19]. The V600E mutation comprises more than 90% of observed BRAF mutations. In subtypes of PTC, the highest rate of BRAF mutation (77%) is found in the tall cell variant of papillary cancer, and the lowest percentage (12%) is found in the FVPTC. In PTC, BRAF mutation is more frequent in older patients, and is associated with extrathyroidal invasion, advanced stage, and worse clinical outcome, including greater rates of recurrence and treatment failure [20,21,22,23]. BRAF mutation is responsible for the suppression of the sodium/iodide symporter (NIS), which is involved in iodine metabolism [24]. By contrast, others have found that the BRAF mutation is not associated with age, gender, multicentricity, recurrence rate, lymphovascular invasion, extranodal extension, central neck involvement, advanced stage (stage III or IV), or distant metastasis [25,26,27].
The most common genetic abnormalities involved in follicular thyroid carcinoma are deletions, partial deletions, and deletion rearrangements of the short arm of chromosome 3. The PAX8/PPAR fusion oncogene occurs via a balanced translocation between chromosomes 2 and 3 that results in the fusion of the DNA-binding domains of the thyroid transcription factor PAX8 to the peroxisome proliferator activated receptor (PPARγ) [3]. The PAX8/PPAR fusion protein (PPFP) is detected in 36.5% of FTCs (range, 11% to 63%), 9.5% of follicular adenomas, and 13.2% of FVPTCs [28]. This chimeric protein may retard the growth inhibition and follicular differentiation normally induced by PPARγ, resulting in tumorigenesis. PPFP is not the only PPARγ fusion mutation that occurs in FTC. Recently, the CREB3L2-PPARγ fusion, which results from a t(3;7)(p25;q34) chromosomal rearrangement, was identified [29].
The treatment of patients with well-differentiated thyroid carcinoma is based on surgery, high-dose radioactive iodine (RAI), and thyroid hormone therapy. However, for patients with advanced, metastatic thyroid carcinoma, treatment options after radioiodine and thyroid hormone therapy have been limited. In patients with radioiodine-resistant disease, cytotoxic chemotherapy yields low response rates of short duration, is often associated with considerable toxicity, and does not prolong survival.
Recently, antiangiogenic therapy has been investigated for the treatment of advanced thyroid cancer. Investigational targeted therapies for WDTC include agents targeting the VEGF pathway and those targeting multiple kinases such as the MAP kinase and PI3 kinase pathways (Table 1) [6]. These inhibitors are typically administered orally and, because of the targeting similarities, common toxicities exist among these agents, including hypertension, diarrhea, skin rashes, and fatigue.
Sorafenib is an oral tyrosine kinase inhibitor (TKI) targeting B-Raf and VEGF receptors 2 and 3. The optimal therapeutic dose of sorafenib is 400 mg twice daily [5]. In a phase II trial, 58 WDTC patients with RAI failure were treated with sorafenib over a 10-month period. Of 41 PTC patients, six had a partial response (PR) and 23 had stable disease lasting longer than 6 months. The median duration of the PR was 7.5 months, the median progression-free survival (PFS) was 15 months, and the thyroglobulin of 77% of PTC patients declined more than 25%. No PRs were noted among non-PTC patients [30]. Another phase II study evaluated the effect of 26 weeks of sorafenib therapy on 31 WDTC patients with metastatic or locally advanced, radioiodine-refractory disease. At the study end, 25% of patients had a PR, 34% had stable disease, and the PFS was 58 weeks. Of 21 patients who underwent radioiodine imaging after 26 weeks of treatment, none had induction of uptake in metastatic lesions [7].
Motesanib is also an oral TKI targeting VEGF receptors 1, 2, and 3. In an open-label, phase II study, 57 PTC patients who had progressive, locally advanced or metastatic, radioiodine-resistant WDTC were treated with 125 mg of motesanib administered orally once daily. The objective response rate was 14%, and stable disease was achieved in 67% of the patients and maintained for 24 weeks or longer in 35%. The median duration of the response was 32 weeks and the median PFS was 40 weeks. Serum thyroglobulin concentrations decreased during treatment in 81% of patients [31].
Sunitinib is approved for the treatment of renal cell carcinoma and is a TKI targeting all three VEGF receptors, RET, and RET/PTC subtypes 1 and 3. Prolonged PRs have been described in three patients (with PTC, FTC, and MTC, respectively) treated with 50 mg of sunitinib daily for 28 days followed by 14 days without treatment per cycle. Fluoro-2-deoxy-D-glucose uptake by positron emission tomography imaging was markedly reduced in the WDTC patients [32]. An open-label phase II trial with sunitinib in 35 patients with progressive WDTC or MTC reported a PR in 13% of 31 WDTC patients, and disease stabilization in 68% of WDTC and 83% of MTC patients [33].
Although the above TKIs targeting the VEGF receptors also inhibit RET, axitinib, and pazopanib do not have any anti-RET activity [34,35]. Axitinib and pazopanib are efficacious in thyroid carcinoma despite the absence of inhibitory activity against RET or other mutated kinases that are oncogenic in thyroid carcinoma, suggesting that VEGF receptor-mediated angiogenesis is likely the primary mechanism by which the other anti-VEGF receptor inhibitory agents function.
Thalidomide was found to inhibit angiogenesis, but the exact mechanism by which thalidomide exerts its antiangiogenic effects remains unknown. An open-label, phase II trial was initiated to examine the efficacy of thalidomide in 36 patients with progressive, metastatic thyroid carcinoma [36]. Starting at 200 mg daily, the dose of thalidomide was progressively increased as tolerated, with a median maximum daily dose of 600 mg. PRs were achieved in 18% of patients, and 32% had stable disease as their best response. Common adverse events included somnolence, peripheral neuropathy, constipation, dizziness, and infection.
Cyclooxygenase-2 (COX-2) is overexpressed in many cancers and promotes tumor development and progression. COX-2 mRNA and protein levels are increased in thyroid cancer compared with nonneoplastic and benign thyroid tissues, especially those expressing RET/PTC mutations. One phase II trial was performed in 32 patients with progressive WDTC treated with celecoxib 400 mg orally twice a day for 12 months. One patient had a PR, and one remained stable on therapy for >12 months, but most patients progressed despite treatment. The study was terminated as a result of a lack of efficacy combined with increasing concern about cardiovascular toxicity from COX-2 inhibitors [37].
Rosiglitazone, a PPARγ agonist, has been shown to induce differentiation, cell cycle arrest, and apoptosis in a variety of human cancers, including thyroid carcinoma. Ten patients with radioiodine scan-negative metastatic WDTC were treated with rosiglitazone at 4 mg daily for 1 week, and then 8 mg daily for 7 weeks. Of the ten patients, four had positive radioiodine scans after rosiglitazone therapy, with uptake in the neck in three patients and in the pelvis in one patient. The serum thyroglobulin level decreased in two patients, increased in five patients, and was stable in three patients. No patient developed clinically important toxicity associated with rosiglitazone treatment.
Papillary thyroid carcinomas, follicular thyroid carcinomas, and their subtypes represent the majority of WDTCs. Mutations in RET/PTC, BRAF, RAS, and PAX8/PPARγ are commonly associated with well-differentiated thyroid carcinoma and may carry diagnostic and prognostic significance. These molecular markers are reflected in the Revised Management Guidelines for Patients with Thyroid Nodules and Differentiated Thyroid Cancer, published by the American Thyroid Association [38]. VEGF- and RET-directed therapies such as sorafenib, motesanib, and sunitinib, novel therapeutic agents targeting the above genetic mutations, have been shown to be the most effective at inducing clinical responses and stabilizing the disease process. In the near future, targeted therapy and the use of tumor biomarkers for predicting responses will be the next step in improving the survival of a disease that not long ago was untreatable.
Figures and Tables
Fig. 1
MAP kinase pathway and thyroid carcinoma. RTK, receptor tyrosine kinase; TRK, tropomyosin-receptor-kinase; RAS, rat sarcoma; RET/PTC, rearranged during transfection/papillary thyroid cancer; BRAF, v-raf murine sarcoma viral oncogene homolog B1; MEK, mitogen-activated protein kinase; ERK, extracellular signal-related kinase.
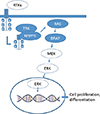
Table 1
Multi-Kinase Inhibitors and Key Targets

Adapted from Gild et al. Nat Rev Endocrinol 2011;7:617-24, with permission from Nature Publishing Group [6].
VEGFR, vascular endothelial growth factor receptor; RET, rearranged during transfection; PDGFR, platelet-derived growth factor receptor; BRAF, v-raf murine sarcoma viral oncogene homolog B1; KIT, v-kit Hardy-Zuckerman 4 feline sarcoma viral oncogene homolog; FLT3, fms-related tyrosine kinase 3; CSF1R, colony stimulating factor 1 receptor; MET, c-Met, also called hepatocyte growth factor receptor; EGFR, endothelial growth factor receptor; FGFR, fibroblast growth factor receptor.
References
1. Cohen Y, Xing M, Mambo E, Guo Z, Wu G, Trink B, Beller U, Westra WH, Ladenson PW, Sidransky D. BRAF mutation in papillary thyroid carcinoma. J Natl Cancer Inst. 2003; 95:625–627.
2. Fusco A, Santoro M, Grieco M, Carlomagno F, Dathan N, Fabien N, Berlingieri MT, Li Z, De Franciscis V, Salvatore D, Melillo RM, Portella G, Cerrato A, Colantuoni V, Vecchio G. RET/PTC activation in human thyroid carcinomas. J Endocrinol Invest. 1995; 18:127–129.
3. Kroll TG, Sarraf P, Pecciarini L, Chen CJ, Mueller E, Spiegelman BM, Fletcher JA. PAX8-PPARgamma1 fusion oncogene in human thyroid carcinoma [corrected]. Science. 2000; 289:1357–1360.
4. Hilger RA, Scheulen ME, Strumberg D. The Ras-Raf-MEK-ERK pathway in the treatment of cancer. Onkologie. 2002; 25:511–518.
5. Wilhelm SM, Carter C, Tang L, Wilkie D, McNabola A, Rong H, Chen C, Zhang X, Vincent P, McHugh M, Cao Y, Shujath J, Gawlak S, Eveleigh D, Rowley B, Liu L, Adnane L, Lynch M, Auclair D, Taylor I, Gedrich R, Voznesensky A, Riedl B, Post LE, Bollag G, Trail PA. BAY 43-9006 exhibits broad spectrum oral antitumor activity and targets the RAF/MEK/ERK pathway and receptor tyrosine kinases involved in tumor progression and angiogenesis. Cancer Res. 2004; 64:7099–7109.
6. Gild ML, Bullock M, Robinson BG, Clifton-Bligh R. Multikinase inhibitors: a new option for the treatment of thyroid cancer. Nat Rev Endocrinol. 2011; 7:617–624.
7. Hoftijzer H, Heemstra KA, Morreau H, Stokkel MP, Corssmit EP, Gelderblom H, Weijers K, Pereira AM, Huijberts M, Kapiteijn E, Romijn JA, Smit JW. Beneficial effects of sorafenib on tumor progression, but not on radioiodine uptake, in patients with differentiated thyroid carcinoma. Eur J Endocrinol. 2009; 161:923–931.
8. Kebebew E, Peng M, Reiff E, Treseler P, Woeber KA, Clark OH, Greenspan FS, Lindsay S, Duh QY, Morita E. A phase II trial of rosiglitazone in patients with thyroglobulin-positive and radioiodine-negative differentiated thyroid cancer. Surgery. 2006; 140:960–966.
9. Nikiforov YE. RET/PTC rearrangement in thyroid tumors. Endocr Pathol. 2002; 13:3–16.
10. Vecchio G, Santoro M. Oncogenes and thyroid cancer. Clin Chem Lab Med. 2000; 38:113–116.
11. Musholt TJ, Musholt PB, Khaladj N, Schulz D, Scheumann GF, Klempnauer J. Prognostic significance of RET and NTRK1 rearrangements in sporadic papillary thyroid carcinoma. Surgery. 2000; 128:984–993.
12. Santoro M, Thomas GA, Vecchio G, Williams GH, Fusco A, Chiappetta G, Pozcharskaya V, Bogdanova TI, Demidchik EP, Cherstvoy ED, Voscoboinik L, Tronko ND, Carss A, Bunnell H, Tonnachera M, Parma J, Dumont JE, Keller G, Hofler H, Williams ED. Gene rearrangement and Chernobyl related thyroid cancers. Br J Cancer. 2000; 82:315–322.
13. Vasko V, Ferrand M, Di Cristofaro J, Carayon P, Henry JF, de Micco C. Specific pattern of RAS oncogene mutations in follicular thyroid tumors. J Clin Endocrinol Metab. 2003; 88:2745–2752.
14. Zhu Z, Gandhi M, Nikiforova MN, Fischer AH, Nikiforov YE. Molecular profile and clinical-pathologic features of the follicular variant of papillary thyroid carcinoma. An unusually high prevalence of ras mutations. Am J Clin Pathol. 2003; 120:71–77.
15. Hagemann C, Rapp UR. Isotype-specific functions of Raf kinases. Exp Cell Res. 1999; 253:34–46.
16. Davies H, Bignell GR, Cox C, Stephens P, Edkins S, Clegg S, Teague J, Woffendin H, Garnett MJ, Bottomley W, Davis N, Dicks E, Ewing R, Floyd Y, Gray K, Hall S, Hawes R, Hughes J, Kosmidou V, Menzies A, Mould C, Parker A, Stevens C, Watt S, Hooper S, Wilson R, Jayatilake H, Gusterson BA, Cooper C, Shipley J, Hargrave D, Pritchard-Jones K, Maitland N, Chenevix-Trench G, Riggins GJ, Bigner DD, Palmieri G, Cossu A, Flanagan A, Nicholson A, Ho JW, Leung SY, Yuen ST, Weber BL, Seigler HF, Darrow TL, Paterson H, Marais R, Marshall CJ, Wooster R, Stratton MR, Futreal PA. Mutations of the BRAF gene in human cancer. Nature. 2002; 417:949–954.
17. Garnett MJ, Marais R. Guilty as charged: B-RAF is a human oncogene. Cancer Cell. 2004; 6:313–319.
18. Kimura ET, Nikiforova MN, Zhu Z, Knauf JA, Nikiforov YE, Fagin JA. High prevalence of BRAF mutations in thyroid cancer: genetic evidence for constitutive activation of the RET/PTC-RAS-BRAF signaling pathway in papillary thyroid carcinoma. Cancer Res. 2003; 63:1454–1457.
19. Xing M, Vasko V, Tallini G, Larin A, Wu G, Udelsman R, Ringel MD, Ladenson PW, Sidransky D. BRAF T1796A transversion mutation in various thyroid neoplasms. J Clin Endocrinol Metab. 2004; 89:1365–1368.
20. Cantwell-Dorris ER, O'Leary JJ, Sheils OM. BRAFV600E: implications for carcinogenesis and molecular therapy. Mol Cancer Ther. 2011; 10:385–394.
21. Palona I, Namba H, Mitsutake N, Starenki D, Podtcheko A, Sedliarou I, Ohtsuru A, Saenko V, Nagayama Y, Umezawa K, Yamashita S. BRAFV600E promotes invasiveness of thyroid cancer cells through nuclear factor kappaB activation. Endocrinology. 2006; 147:5699–5707.
22. Xing M, Westra WH, Tufano RP, Cohen Y, Rosenbaum E, Rhoden KJ, Carson KA, Vasko V, Larin A, Tallini G, Tolaney S, Holt EH, Hui P, Umbricht CB, Basaria S, Ewertz M, Tufaro AP, Califano JA, Ringel MD, Zeiger MA, Sidransky D, Ladenson PW. BRAF mutation predicts a poorer clinical prognosis for papillary thyroid cancer. J Clin Endocrinol Metab. 2005; 90:6373–6379.
23. Caronia LM, Phay JE, Shah MH. Role of BRAF in thyroid oncogenesis. Clin Cancer Res. 2011; 17:7511–7517.
24. Durante C, Puxeddu E, Ferretti E, Morisi R, Moretti S, Bruno R, Barbi F, Avenia N, Scipioni A, Verrienti A, Tosi E, Cavaliere A, Gulino A, Filetti S, Russo D. BRAF mutations in papillary thyroid carcinomas inhibit genes involved in iodine metabolism. J Clin Endocrinol Metab. 2007; 92:2840–2843.
25. Fugazzola L, Mannavola D, Cirello V, Vannucchi G, Muzza M, Vicentini L, Beck-Peccoz P. BRAF mutations in an Italian cohort of thyroid cancers. Clin Endocrinol (Oxf). 2004; 61:239–243.
26. Gouveia C, Can NT, Bostrom A, Grenert JP, van Zante A, Orloff LA. Lack of association of BRAF mutation with negative prognostic indicators in papillary thyroid carcinoma: the University of California, San Francisco, experience. JAMA Otolaryngol Head Neck Surg. 2013; 139:1164–1170.
27. Puxeddu E, Moretti S, Giannico A, Martinelli M, Marino C, Avenia N, Cristofani R, Farabi R, Reboldi G, Ribacchi R, Pontecorvi A, Santeusanio F. Ret/PTC activation does not influence clinical and pathological features of adult papillary thyroid carcinomas. Eur J Endocrinol. 2003; 148:505–513.
28. Nikiforova MN, Lynch RA, Biddinger PW, Alexander EK, Dorn GW 2nd, Tallini G, Kroll TG, Nikiforov YE. RAS point mutations and PAX8-PPAR gamma rearrangement in thyroid tumors: evidence for distinct molecular pathways in thyroid follicular carcinoma. J Clin Endocrinol Metab. 2003; 88:2318–2326.
29. Lui WO, Zeng L, Rehrmann V, Deshpande S, Tretiakova M, Kaplan EL, Leibiger I, Leibiger B, Enberg U, Hoog A, Larsson C, Kroll TG. CREB3L2-PPARgamma fusion mutation identifies a thyroid signaling pathway regulated by intramembrane proteolysis. Cancer Res. 2008; 68:7156–7164.
30. Kloos RT, Ringel MD, Knopp MV, Hall NC, King M, Stevens R, Liang J, Wakely PE Jr, Vasko VV, Saji M, Rittenberry J, Wei L, Arbogast D, Collamore M, Wright JJ, Grever M, Shah MH. Phase II trial of sorafenib in metastatic thyroid cancer. J Clin Oncol. 2009; 27:1675–1684.
31. Sherman SI, Wirth LJ, Droz JP, Hofmann M, Bastholt L, Martins RG, Licitra L, Eschenberg MJ, Sun YN, Juan T, Stepan DE, Schlumberger MJ. Motesanib Thyroid Cancer Study Group. Motesanib diphosphate in progressive differentiated thyroid cancer. N Engl J Med. 2008; 359:31–42.
32. Dawson SJ, Conus NM, Toner GC, Raleigh JM, Hicks RJ, McArthur G, Rischin D. Sustained clinical responses to tyrosine kinase inhibitor sunitinib in thyroid carcinoma. Anticancer Drugs. 2008; 19:547–552.
33. Carr LL, Mankoff DA, Goulart BH, Eaton KD, Capell PT, Kell EM, Bauman JE, Martins RG. Phase II study of daily sunitinib in FDG-PET-positive, iodine-refractory differentiated thyroid cancer and metastatic medullary carcinoma of the thyroid with functional imaging correlation. Clin Cancer Res. 2010; 16:5260–5268.
34. Inai T, Mancuso M, Hashizume H, Baffert F, Haskell A, Baluk P, Hu-Lowe DD, Shalinsky DR, Thurston G, Yancopoulos GD, McDonald DM. Inhibition of vascular endothelial growth factor (VEGF) signaling in cancer causes loss of endothelial fenestrations, regression of tumor vessels, and appearance of basement membrane ghosts. Am J Pathol. 2004; 165:35–52.
35. Bible KC, Suman VJ, Menefee ME, Smallridge RC, Molina JR, Maples WJ, Karlin NJ, Traynor AM, Kumar P, Goh BC, Lim WT, Bossou AR, Isham CR, Webster KP, Kukla AK, Bieber C, Burton JK, Harris P, Erlichman C. Mayo Phase 2 Consortium. Mayo Clinic Endocrine Malignances Disease Oriented Group. A multiinstitutional phase 2 trial of pazopanib monotherapy in advanced anaplastic thyroid cancer. J Clin Endocrinol Metab. 2012; 97:3179–3184.
36. Ain KB, Lee C, Williams KD. Phase II trial of thalidomide for therapy of radioiodine-unresponsive and rapidly progressive thyroid carcinomas. Thyroid. 2007; 17:663–670.
37. Mrozek E, Kloos RT, Ringel MD, Kresty L, Snider P, Arbogast D, Kies M, Munden R, Busaidy N, Klein MJ, Sherman SI, Shah MH. Phase II study of celecoxib in metastatic differentiated thyroid carcinoma. J Clin Endocrinol Metab. 2006; 91:2201–2204.
38. American Thyroid Association (ATA) Guidelines Taskforce on Thyroid Nodules and Differentiated Thyroid Cancer. Cooper DS, Doherty GM, Haugen BR, Kloos RT, Lee SL, Mandel SJ, Mazzaferri EL, McIver B, Pacini F, Schlumberger M, Sherman SI, Steward DL, Tuttle RM. Revised American Thyroid Association management guidelines for patients with thyroid nodules and differentiated thyroid cancer. Thyroid. 2009; 19:1167–1214.




 PDF
PDF ePub
ePub Citation
Citation Print
Print


 XML Download
XML Download