There have been four major events over the last century that have contributed to the better understanding and treatment of primary hyperparathyroidism: the routine measurement of serum calcium with recognition of hypercalcemia, the secured diagnosis with measurement of elevated parathyroid hormone (PTH) levels, the preoperative localization of hypersecreting parathyroid gland(s), and the use of intraoperative measurement of PTH dynamics. Targeted parathyroidectomy guided by intraoperative parathyroid hormone monitoring (IPM) through a small cervical incision has replaced traditional bilateral neck exploration (BNE) as the initial approach in the surgical treatment of patients with primary hyperparathyroidism at many medical centers worldwide. Preoperative Tc-99m-sestamibi localization scans and/or ultrasound serve as an important prerequisite for successful targeted parathyroidectomy. Operative success rates are further improved by IPM before and after removal of all abnormal parathyroid glands. When intraoperative PTH levels are reduced by more than 50% from the highest pre-incision or pre-excision PTH level after resection of an abnormal gland, successful operative resection is assured with a predictive cure rate of > 95%. The advantages of targeted parathyroidectomy in the ambulatory setting include improved cosmetic results, decreased postoperative pain, shorter operative time, decreased hospitalization and rapid postoperative recovery with a success rate comparable to BNE. This article reviews the current understanding and treatment of primary hyperparathyroidism.
Primary hyperparathyroidism results from an overproduction of PTH by one or more hyperfunctioning parathyroid glands, which usually leads to hypercalcemia. Humans typically have 4 parathyroid glands (84%), but may have 5 or more (supernumerary) glands (13%) or only 3 parathyroid glands (3%) [1]. The incidence of primary hyperparathyroidism increases with age, and ranges from 0.1-0.3% [2]. Primary hyperparathyroidism occurs more frequently in women than men with a ratio of 3:1. Parathyroid adenoma is a benign encapsulated tumor that accounts for the majority of cases (85-96%) of primary hyperparathyroidism. Although most have a single affected gland, 2-5% of patients with primary hyperparathyroidism may have more than one affected parathyroid gland. Parathyroid hyperplasia is caused by an increase of parenchymal mass within the parathyroid glands, and it accounts for 4-15% of cases. The incidence of parathyroid hyperplasia increases in patients with multiple endocrine neoplasia (MEN) types 1 and 2, and non-MEN familial isolated hyperparathyroidism (FIHPT). Parathyroid hyperplasia is treated by either subtotal parathyroidectomy (3½ glands removed) or total parathyroidectomy with autotransplantation. For patients with MEN, cervical thymectomy should also be performed. Parathyroid carcinoma is an indolent growing malignant tumor responsible for less than 5% of all primary hyperparathyroidism cases. Since this carcinoma is invasive, parathyroidectomy should be performed with en bloc resection of the ipsilateral thyroid lobe avoiding parathyroid capsule violation.
The clinical presentation of primary hyperparathyroidism has evolved throughout the years. The classic description of kidney "stones", painful "bones", abdominal "groans", lethargic "moans" and psychiatric "overtones" are now infrequently encountered in developed countries. With the widespread use of serum channel autoanalyzers since the 1960's, patients with primary hyperparathyroidism most commonly present with abnormal biochemical results before the manifestation of clinical symptoms. At this earlier stage of diagnosis, most patients found to have primary hyperparathyroidism are asymptomatic (75-80%) with the disease being evident only as hypercalcemia on routine chemistry analysis [3,4].
The diagnosis of primary hyperparathyroidism is confirmed by demonstrating high serum calcium levels, and elevated intact PTH levels in the setting of normal renal function. The development of an assay to measure human PTH in 1968 has since enabled the more accurate diagnosis of primary hyperparathyroidism. Evidence of increased vitamin D metabolism, secondary to increased intact PTH, can also be seen in patients with primary hyperparathyroidism, and is typically characterized by low plasma levels of 25-hydroxyvitamin D and high plasma levels of 1,25-dihydroxyvitamin D. Twenty-four hour urine calcium collection is also helpful to exclude the diagnosis of benign familial hypocalciuric hypercalcemia (BFHH). Patients with this rare condition usually have a family history of hypercalcemia and decreased urine calcium excretion since birth. BFHH biochemically mimics primary hyperparathyroidism with elevated calcium and PTH levels, but with low levels of urinary calcium (less than 100 mg/24 hours). Similarly, the urinary calcium-to-creatinine (Ca/Cr) clearance ratio in BFHH is less than 0.01; whereas in patients with primary hyperparathyroidism, the ratio is greater than 0.02. An autosomal dominant disease, BFHH is a benign condition not corrected by parathyroidectomy. Other causes of hypercalcemia that should be excluded include underlying malignancy, excessive thiazide diuretics, lithium use, vitamin A or D excess, milk-alkali syndrome, hyper- or hypothyroidism, sarcoidosis, Paget's disease, prolonged immobilization, dehydration and granulomatous diseases.
Some patients may have primary hyperparathyroidism with normal calcium and elevated PTH levels after appropriate diagnostic evaluation. This condition known as normocalcemic hyperparathyroidism is generally recognized in patients being evaluated for osteoporosis [5]. In some individuals, this may be an early presentation of symptomatic primary hyperparathyroidism; whereas in others, it may represent PTH elevation secondary to vitamin D deficiency or renal dysfunction [6]. Both of these conditions result in decreased levels of 1,25-dihydroxyvitamin D, which would otherwise serve to increase serum calcium and phosphate by increasing absorption from the GI tract, renal reabsorption of calcium and bone resorption of calcium and phosphate. Since it also serves to inhibit PTH secretion, a deficiency would lead to increased levels of PTH. On other occasions, patients will present with hypercalcemia and inappropriately normal PTH levels, and after further evaluation, will have primary hyperparathyroidism. Nevertheless, physicians managing and treating primary hyperparathyroidism should be knowledgeable about its varied presentations in order to avoid poor surgical outcomes.
Although not required for diagnosis, dual-energy X-ray absorptiometry (DEXA) may be useful in the measurement of decreased bone mineral density (BMD) in patients with primary hyperparathyroidism. If osteoporosis is documented, as evidenced by T-score (less than -2.5), parathyroidectomy should be recommended. In primary hyperparathyroidism, bone density losses are greater in areas of cortical bone such as the femoral neck and radius, but can occur at all bony sites. The benefit of parathyroidectomy is more pronounced at the hips because of the morbidity and mortality associated with bone fractures at these sites.
Parathyroidectomy is clearly indicated in those patients with symptomatic primary hyperparathyroidism. Patients with typical bone, renal, gastrointestinal or neuromuscular symptoms, as well as life-threatening hypercalcemia are surgical candidates. However, there remains some controversy among clinicians regarding the indications for performing parathyroidectomy in asymptomatic patients. A long-term study of patients with primary hyperparathyroidism over 15 years suggests that previous National Institutes of Health (NIH) guidelines for parathyroidectomy do not reliably predict worsening disease progression in asymptomatic patients [7]. In an attempt to address this ongoing debate, these recommendations for surgical treatment of asymptomatic patients previously established by the NIH were modified at the Third International Workshop on the Management of Asymptomatic Primary Hyperparathyroidism [8,9] (Table 1). Although some surgeons rely on these objective criteria, experienced parathyroid surgeons believe that subjective complaints also warrant operative intervention. Others now believe that neurocognitive deficits are indications for operative intervention in patients with primary hyperparathyroidism. This stems from a number of studies that show improvement of weakness, fatigability, bone pain, joint pain, mood swings, and poor memory after parathyroidectomy [10]. Further evidence suggests that there is a significant decrease in the risk of bone fracture in all patients with hyperparathyroidism following parathyroidectomy [11]. Nevertheless, all "asymptomatic" patients with clear biochemical diagnosis of primary hyperparathyroidism should be offered a surgical consultation with an experienced parathyroid surgeon.
Over the years, emerging imaging modalities and techniques have considerably improved the preoperative localization of abnormal parathyroid glands. The success of targeted parathyroidectomy is largely predicated on the accuracy of these preoperative studies in the localization of hypersecreting parathyroid glands. Nevertheless, while helpful to the surgeon in performing targeted parathyroidectomy, localization studies should not be used to make the diagnosis of primary hyperparathyroidism. Furthermore, selection of the most appropriate imaging study requires an understanding of the accuracy of each modality, particularly at one's institution, in addition to many other factors that may improve or limit its accuracy.
Sestamibi scintigraphy plays an important role in the preoperative localization of abnormal parathyroid glands in targeted parathyroidectomy for primary hyperparathyroidism (Fig. 1). Sestamibi can localize 80-90% of single abnormal parathyroid glands, but it is less sensitive in the diagnosis of multiglandular disease (MGD) [12-14]. The specificity of sestamibi scanning can be improved with delayed (two-hour) planar and multi-dimensional imaging obtained by single photon emission computed tomography (SPECT) [15,16]. Although there are no significant differences in the sensitivities of the varied types of sestamibi scanning used to identify abnormal gland(s), multidimensional imaging (i.e. SPECT) can provide additional information regarding the anatomical location of these glands in certain patients [17]. Despite the fact that its sensitivity for MGD is low, misdiagnosis can be avoided with additional preoperative imaging and/or IPM [13,14,18]. The co-existence of thyroid nodules or lymph nodes that can mimic abnormal parathyroid glands may also cause false-positive results on sestamibi scans. Such results can be minimized by combining sestamibi with neck ultrasound to distinguish between thyroid lesions and enlarged parathyroid glands. Another option is the combined use of SPECT/CT that can provide simultaneous three-dimensional functional and anatomical information delineating thyroid and parathyroid lesions [19,20] (Fig. 2). Sestamibi-SPECT is particularly useful in detecting smaller parathyroid lesions that may reside posterior to the thyroid gland.
Ultrasound (US) has recently been more frequently used for preoperative parathyroid localization. This imaging modality can be very accurate as it can provide important anatomical information delineating an enlarged parathyroid gland from surrounding structures with 70-80% accuracy [21,22]. This non-invasive imaging modality is inexpensive, but has varied sensitivity in the localization of abnormal parathyroid glands. Furthermore, US is operator dependent with limited application to the neck for parathyroid localization since it cannot identify ectopic glands in the mediastinum. At many institutions, US is routinely performed by parathyroid surgeons in the clinic setting. Surgeon-performed ultrasound (SUS) enables these specialists who best appreciate neck anatomy to obtain real time information regarding the anatomical location of enlarged parathyroid glands among several other structures, and allows for evaluation of thyroid abnormalities that may require surgical intervention (Fig. 3). If performed by a surgeon prior to parathyroidectomy, ultrasound can improve localization of the abnormal gland from 80% to 93% when the sestamibi scan does not localize the gland [23]. In fact, several studies demonstrate that preoperative SUS localizes enlarged parathyroid glands similar to sestamibi with improved sensitivity over radiologist-performed ultrasound [21-23]. Like sestamibi, US has a lower sensitivity for MGD. The overall sensitivity of dual-phase 99mTc sestamibi scintigraphy in comparison to US is 88% versus 79% for single adenomas, 30% versus 16% for double adenomas, and 44% versus 35% MGD respectively [24].
Combined sestamibi and US can increase the accuracy of localization of a single adenoma from 94% to 99%. When concordant, sestamibi and US localization has been reported to have an operative success rate approaching 99%, obviating the need for IPM [25-28]. While studies show excellent outcomes in this subgroup of highly selected patients with concordant localizing studies, this selective approach significantly limits the number of eligible patients for targeted parathyroidectomy. Preoperative sestamibi and US have been shown to be concordant only 50-60% of the time, thereby leaving a great number of patients with no definitive or discordant localization [29]. Discordance between sestamibi and US has been reported to be as high as 38% in consecutive patients treated by parathyroidectomy, with an 11% rate of MGD. Although the sensitivities for both localizing studies for MGD are lower, the risk of missing abnormal glands can be minimized by utilizing additional intraoperative PTH monitoring (IPM) [29].
In patients with recurrent or persistent disease, 4D-CT may have a useful role in the localization of abnormal parathyroid glands in conjunction with other imaging studies [30]. The wash-in and wash-out characteristics of parathyroid glands allow 4D-CT to identify hyperfunctioning glands with improved sensitivity. Although under current investigation for routine preoperative parathyroid localization, 4D-CT remains a useful imaging modality in re-operative patients.
An important innovation in the surgical treatment of primary hyperparathyroidism, IPM serves as a surgical adjunct to quantitatively confirm the excision of all hyperfunctioning parathyroid tissue. Refined and first implemented routinely by Dr. George Irvin at the University of Miami, IPM allows for limited or minimally invasive operations that obviate the need to identify all 4 parathyroid glands [31,32]. IPM can confirm adequate removal of all hyperfunctioning parathyroid glands and predict operative success with reduced operative time when compared to BNE. A paradigm shift in the surgical management of primary hyperparathyroidism, abnormal hypersecretory activity of parathyroid glands is determined exclusively by measured PTH levels, not by gland size or histopathology. The success and adequacy of parathyroidectomy, therefore, is primarily determined by the function and not form (size/histopathology) of parathyroid glands. Patients with more than one hypersecreting parathyroid gland by IPM measurement are determined to have MGD. IPM is possible due to the short half-life (3.5 to 5 min) of PTH. IPM involves the rapid assay measurement of PTH levels pre-incision, pre-excision, and post excision at 5 and 10 minutes after removal of all hypersecreting parathyroid glands. Originally described by Irvin, the intraoperative criterion for successful and curative parathyroidectomy is a decrease of intact PTH levels by greater than 50% from the highest pre-incision or pre-excision hormone level in a peripheral blood sample obtained 10 minutes after removal of all abnormal parathyroid tissue [31-33]. If this criterion is met, the operation is completed and the incision is closed (Fig. 4). If the 10 minute sample shows an insufficient decrease, further neck exploration for additional hypersecreting parathyroid glands is performed.
A PTH level decline of greater than 50% from the highest pre-incision or pre-excision level indicates operative success with predictive cure in 97% of cases [33]. In spite of this, other surgeons use more stringent intraoperative criteria to determine operative cure of primary hyperparathyroidism. In addition to a greater than 50% reduction of intraoperative PTH level, these proponents require that the final post-excision PTH level fall into the normal range before concluding the surgical procedure in an attempt to lower the incidence of operative failure or persistent hyperparathyroidism secondary to false positive results [34,35]. Other evidence, however, suggests that patients in whom intraoperative PTH levels decreased by greater than 50% but did not drop into normal range continue to be biochemically eucalcemic after the operation without an increased incidence of operative failure or recurrent hyperparathyroidism [36].
In patients with discordant or incorrect concordant preoperative localization studies, IPM remains a useful adjunct that limits exploration and prevents operative failure due to MGD [29]. When both sestamibi and US studies are negative, differential venous PTH sampling measured by rapid assay can be performed to lateralize the hypersecreting gland to one side of the neck [37,38]. If this test is indeterminate, one side is randomly chosen to be explored first. Operative failure and BNE rates have decreased significantly for initial and re-operative parathyroidectomy with the advent of IPM. Failure rates from initial parathyroidectomy have reportedly decreased by 4.5% after the implementation of IPM [39]. Although variable, IPM has been reported to increase success rates by 10% at initial operation, and by 18% in re-operative patients for failed parathyroidectomy [40,41].
Targeted parathyroidectomy is performed under general or local anesthesia in the ambulatory setting. Prior to limited exploration through a 2-4 cm cervical incision, a peripheral blood sample for a baseline (pre-incision) PTH level is obtained. After identification of the abnormal parathyroid gland and just prior to clamping of its vascular pedicle, a pre-excision PTH hormone level is measured. Once the abnormal gland is excised, PTH levels are measured by subsequent 5 and 10 minute blood samples. As previously mentioned, if the 10 minutes post-excision PTH level is reduced by greater than 50%, the procedure is completed and the incision is closed (Fig. 4). However, if there is insufficient decrease, further neck exploration is performed for additional abnormal parathyroid glands (i.e. MGD). Patients are carefully followed in the outpatient setting with serum calcium, PTH and vitamin D levels measured at 2 months, 6 months and annually thereafter to determine operative outcome. Operative success can be defined as continuous eucalcemia lasting 6 months or longer. Recurrent hyperparathyroidism is defined as elevated calcium and PTH levels more than 6 months after successful parathyroidectomy. Finally, operative failure is defined as elevated calcium and PTH levels within 6 months after parathyroidectomy.
Several studies confirm the success of targeted parathyroidectomy with rates comparable to traditional BNE [40,42-45]. In one study of 656 consecutive patients over 11 years of which 255 underwent targeted parathyroidectomy and 401 conventional BNE, the success rates were 99% and 97% with complication rates of 1.2% and 3% respectively [42]. Targeted parathyroidectomy also had a reduced operating time of 1.3 hours compared to 2.4 hours for BNE, and a reduction in length of hospitalization of 0.24 days compared to 1.64 days respectively. In another subsequent study of 718 patients over 34 years, the operative success rates for targeted parathyroidectomy and BNE were 97% and 94% with operative failure rates of 3% and 6% respectively [44]. Additionally, patients who underwent targeted parathyroidectomy had a lower incidence of MGD by 7% compared to the BNE group. Finally, in a 5-year follow-up of a randomized controlled trial, targeted parathyroidectomy provided the same long-term results as traditional BNE in patients with primary hyperparathyroidism [46]. The aforementioned studies all concluded that targeted parathyroidectomy was a valid and attractive alternative to BNE for most patients with primary hyperparathyroidism.
With limited long-term data to confirm the durability of operative success from targeted parathyroidectomy compared to conventional BNE, critics of the limited approach predict that the combined use of preoperative localization and IPM will miss additional abnormal glands (i.e. MGD) leading to future disease recurrence. In one study of simulated targeted parathyroidectomy in 916 patients with primary hyperparathyroidism, preoperative sestamibi and ultrasound were used for parathyroid localization, and IPM was performed in all patients [47]. Routine BNE was then performed in all these patients revealing 16% having additional enlarged parathyroid glands. The authors concluded that the long term failure or recurrence rate for targeted parathyroidectomy may be higher than reported in early results [47]. What remains unknown is whether or not these enlarged parathyroid glands found on BNE that are judged to be abnormal by the surgeon and may otherwise been left alone or never seen during targeted operations later become hyperfunctioning glands.
There are reports, however, that suggest otherwise demonstrating that targeted parathyroidectomy has a long-term durability of operative success comparable to BNE. In one study with a mean follow-up of almost 5 years, none of the 181 patients cured by image-guided parathyroidectomy developed recurrent disease [48]. In a randomized clinical trial with a 5 year follow-up, the recurrence rates for focused parathyroidectomy and BNE were 5% and 3% respectively [46]. In yet another analysis from the author's institution, a 3% disease recurrence rate developed in 164 patients with a mean follow-up of almost 7 years after successful targeted parathyroidectomy with IPM [49]. Many other studies demonstrate that parathyroid size or histopathology do not necessarily correlate with PTH secretion and therefore may not be reliable indices for determining abnormal hypersecreting parathyroid glands [50-52]. These findings together indicate that the targeted approach has a durable rate of operative success, does not fail to identify MGD as a cause of recurrent hyperparathyroidism, and strongly suggest that normally secreting, though variably sized parathyroid glands left in situ, do not contribute to higher long term failure or recurrence rates.
The traditional approach in the surgical treatment of primary hyperparathyroidism, BNE requires the identification and careful examination of usually all 4 parathyroid glands. When performed by experienced surgeons, the operative cure rate for BNE is greater than 95% with a complication rate ranging from 1-4% [53,54]. There are certain clinical conditions where BNE is preferred over targeted parathyroidectomy. BNE is indicated for cases of MEN and non-MEN familial isolated hyperparathyroidism where there is a higher incidence of parathyroid hyperplasia rather than single gland disease. Due to its higher incidence of MGD, lithium-associated hyperparathyroidism is another condition where BNE is preferable. When patients have more than one gland localized on preoperative studies or no localization, BNE should be considered. BNE is also indicated in patients with secondary or tertiary hyperparathyroidism where parathyroid hyperplasia is most commonly seen. In patients with concomitant thyroid disease requiring both parathyroidectomy and thyroidectomy, as well as in those cases of parathyroid cancer, BNE is performed.
BNE is performed through a 3-5 cm cervical incision under general anesthesia. The procedure involves identification of usually 4 parathyroid glands, removal of the abnormal gland(s) and biopsy of one or more normal parathyroid glands. In cases where more than one parathyroid gland appear abnormal, only the involved glands are excised. IPM can be used as a surgical adjunct to confirm removal of all hypersecreting parathyroid tissue. When not identified initially during BNE, a diligent search for the ectopic abnormal parathyroid gland(s) is performed. This involves exploration of the upper mediastinum, retroesophageal area, carotid sheaths and thyroid gland. If the gland is still not found, the procedure ends. A planned cervical re-exploration, sternotomy or video-assisted mediastinal parathyroid exploration may be performed at a later date after re-evaluation of the initial diagnosis and further imaging to localize a possible unidentified mediastinal gland. In conditions of hyperplasia where all four parathyroid glands appear abnormal, subtotal parathyroidectomy (3½ gland resection) or total parathyroidectomy with autotransplantation is performed. For subtotal parathyroidectomy, the one gland that appears most normal is biopsied and partially resected, leaving a tissue remnant of approximately 50 mg. Once this is completed, the remaining glands are removed. In cases of total parathyroidectomy, remnant parathyroid tissue is autotransplanted, preferably into the brachioradialis muscle of the non-dominant forearm with cryopreservation of the remaining parathyroid tissue. Of note, a bilateral thymectomy is recommended in both surgical approaches due to a high incidence of supernumerary parathyroid glands within these structures.
Recent interest has revolved around the development of minimal access surgical techniques that include endoscopic and video-assisted parathyroidectomy performed with clear preoperative localization as well as IPM to verify the adequacy of abnormal parathyroid gland resection. Both surgical approaches have not been widely adopted, and most parathyroid surgeons do not consider these techniques to be better than the current surgical treatment of primary hyperparathyroidism with targeted parathyroidectomy or BNE [55]. In addition to the long learning curve necessary to gain proficiency in these techniques, they are contraindicated in patients with large thyroid goiters, previous neck surgery, MGD, MEN or non MEN familial hyperparathyroidism, equivocal or negative preoperative localization studies, or parathyroid carcinoma. Nevertheless, surgeons keen on performing endoscopic and/or video-assisted parathyroidectomy should have considerable experience with the conventional approaches of targeted parathyroidectomy and BNE before attempting these minimal access procedures.
Radioguided parathyroidectomy is another surgical approach used in the treatment of primary hyperparathyroidism. Patients are injected with Tc-99m sestamibi isotope approximately two hours before surgery. They are then taken to the operating room where a gamma probe is used to direct the incision site and localize the abnormal parathyroid gland(s) for excision. After the suspected adenoma is removed, the gamma probe is used to measure the radioactivity of the excised tissue, which is then compared to the radioactivity of the surgical bed. Radioguided parathyroidectomy may be useful in re-operative cases, patients who have undergone total thyroidectomy or cases of ectopic parathyroid tissue. IPM can be used as an adjunct to this technique to increase its operative success rate [56,57]. The routine use of radioguided parathyroidectomy has not been widely adopted and most parathyroid surgeons do not use this approach, arguing that the gamma probe does not provide additional useful information that cannot otherwise be obtained with preoperative localization studies and IPM [57-59].
With the advent of routine measurement of serum calcium and recognition of hypercalcemia, the secured diagnosis with measurement of elevated PTH levels, the preoperative localization of abnormal hypersecreting parathyroid gland(s), and the use of intraoperative measurement of PTH dynamics, primary hyperparathyroidism is better understood as functional and metabolic disease manifested by the autonomous secretion of PTH in excess amount. Furthermore, removal of the hypersecreting parathyroid gland and its overproduction of hormone controls the disease both biochemically and clinically. Targeted parathyroidectomy guided by IPM has changed the traditional surgical approach in treating primary hyperparathyroidism. Targeted parathyroidectomy has durable operative success rates of > 95% comparable to BNE, and it can be performed in an ambulatory setting with minimal morbidity. The additional advantages of targeted parathyroidectomy include improved cosmetic results with smaller incisions, decreased postoperative pain, shorter operative time, decreased hospitalization and rapid postoperative recovery. The targeted approach has also served as an impetus for further investigation and development of more minimally invasive parathyroid operations through endoscopic and video-enhanced methods. Despite such advances, however, nothing can replace the sound judgment and experience of a parathyroid surgeon for the successful outcome of these operations.
Figures and Tables
Fig. 1
Coronal tomogram from a delayed phase Sestamibi scan in a patient with a left inferior parathyroid adenoma.
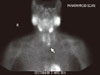
Fig. 2
Delayed phase coronal fused SPECT/CT tomogram showing a left superior parathyroid adenoma (arrow) with a posterior location at the upper pole of the left thyroid lobe.
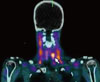
Fig. 3
A. Transverse view of SUS showing a right inferior parathyroid adenoma (arrow). B. Longitudinal view of same right inferior parathyroid adenoma (arrow) with typical ultrasound features including hypoechogenicity and elliptical shape.
Fig. 4
Intraoperative parathyroid hormone monitoring in a patient with a solitary hyperfunctioning parathyroid gland. The 71% intraoperative parathyroid hormone (PTH) drop from 139 pg/mL to 41 pg/mL confirms all abnormal parathyroid tissue has been surgically removed.

References
1. Akerstrom G, Malmaeus J, Bergstrom R. Surgical anatomy of human parathyroid glands. Surgery. 1984. 95:14–21.
2. Uden P, Chan A, Duh QY, Siperstein A, Clark OH. Primary hyperparathyroidism in younger and older patients: symptoms and outcome of surgery. World J Surg. 1992. 16:791–797.
3. Eigelberger MS, Cheah WK, Ituarte PH, Streja L, Duh QY, Clark OH. The NIH criteria for parathyroidectomy in asymptomatic primary hyperparathyroidism: are they too limited? Ann Surg. 2004. 239:528–535.
4. Bilezikian JP, Potts JT Jr. Asymptomatic primary hyperparathyroidism: new issues and new questions--bridging the past with the future. J Bone Miner Res. 2002. 17:Suppl 2. N57–N67.
5. Monchik JM, Gorgun E. Normocalcemic hyperparathyroidism in patients with osteoporosis. Surgery. 2004. 136:1242–1246.
6. Tordjman KM, Greenman Y, Osher E, Shenkerman G, Stern N. Characterization of normocalcemic primary hyperparathyroidism. Am J Med. 2004. 117:861–863.
7. Rubin MR, Bilezikian JP, McMahon DJ, Jacobs T, Shane E, Siris E, Udesky J, Silverberg SJ. The natural history of primary hyperparathyroidism with or without parathyroid surgery after 15 years. J Clin Endocrinol Metab. 2008. 93:3462–3470.
8. Bilezikian JP, Potts JT Jr, Fuleihan Gel H, Kleerekoper M, Neer R, Peacock M, Rastad J, Silverberg SJ, Udelsman R, Wells SA Jr. Summary statement from a workshop on asymptomatic primary hyperparathyroidism: a perspective for the 21st century. J Bone Miner Res. 2002. 17:Suppl 2. N2–N11.
9. Bilezikian JP, Khan AA, Potts JT Jr. Guidelines for the management of asymptomatic primary hyperparathyroidism: summary statement from the third international workshop. J Clin Endocrinol Metab. 2009. 94:335–339.
10. Pasieka JL, Parsons LL. Prospective surgical outcome study of relief of symptoms following surgery in patients with primary hyperparathyroidism. World J Surg. 1998. 22:513–518.
11. VanderWalde LH, Liu IL, O'Connell TX, Haigh PI. The effect of parathyroidectomy on bone fracture risk in patients with primary hyperparathyroidism. Arch Surg. 2006. 141:885–889.
12. Chiu B, Sturgeon C, Angelos P. What is the link between nonlocalizing sestamibi scans, multigland disease, and persistent hypercalcemia? A study of 401 consecutive patients undergoing parathyroidectomy. Surgery. 2006. 140:418–422.
13. Carneiro-Pla DM, Solorzano CC, Irvin GL 3rd. Consequences of targeted parathyroidectomy guided by localization studies without intraoperative parathyroid hormone monitoring. J Am Coll Surg. 2006. 202:715–722.
14. Yip L, Pryma DA, Yim JH, Virji MA, Carty SE, Ogilvie JB. Can a light-bulb sestamibi SPECT accurately predict single-gland disease in sporadic primary hyperparathyroidism? World J Surg. 2008. 32:784–792.
15. Casas AT, Burke GJ, Sathyanarayana , Mansberger AR Jr, Wei JP. Prospective comparison of technetium-99m-sestamibi/iodine-123 radionuclide scan versus high-resolution ultrasonography for the preoperative localization of abnormal parathyroid glands in patients with previously unoperated primary hyperparathyroidism. Am J Surg. 1993. 166:369–373.
16. Caixas A, Berna L, Hernandez A, Tebar FJ, Madariaga P, Vegazo O, Bittini AL, Moreno B, Faure E, Abos D, Piera J, Rodriguez JM, Farrerons J, Puig-Domingo M. Efficacy of preoperative diagnostic imaging localization of technetium 99m-sestamibi scintigraphy in hyperparathyroidism. Surgery. 1997. 121:535–541.
17. Sharma J, Mazzaglia P, Milas M, Berber E, Schuster DM, Halkar R, Siperstein A, Weber CJ. Radionuclide imaging for hyperparathyroidism (HPT): which is the best technetium-99m sestamibi modality? Surgery. 2006. 140:856–863.
18. Anderson SR, Vaughn A, Karakla D, Wadsworth JT. Effectiveness of surgeon interpretation of technetium tc 99m sestamibi scans in localizing parathyroid adenomas. Arch Otolaryngol Head Neck Surg. 2008. 134:953–957.
19. Melton GB, Somervell H, Friedman KP, Zeiger MA, Cahid Civelek A. Interpretation of 99mTc sestamibi parathyroid SPECT scan is improved when read by the surgeon and nuclear medicine physician together. Nucl Med Commun. 2005. 26:633–638.
20. Eslamy HK, Ziessman HA. Parathyroid scintigraphy in patients with primary hyperparathyroidism: 99mTc sestamibi SPECT and SPECT/CT. Radiographics. 2008. 28:1461–1476.
21. Solorzano CC, Carneiro-Pla DM, Irvin GL 3rd. Surgeon-performed ultrasonography as the initial and only localizing study in sporadic primary hyperparathyroidism. J Am Coll Surg. 2006. 202:18–24.
22. Jabiev AA, Lew JI, Solorzano CC. Surgeon-performed ultrasound: a single institution experience in parathyroid localization. Surgery. 2009. 146:569–575.
23. Solorzano CC, Lee TM, Ramirez MC, Carneiro DM, Irvin GL. Surgeon-performed ultrasound improves localization of abnormal parathyroid glands. Am Surg. 2005. 71:557–562.
24. Ruda JM, Hollenbeak CS, Stack BC Jr. A systematic review of the diagnosis and treatment of primary hyperparathyroidism from 1995 to 2003. Otolaryngol Head Neck Surg. 2005. 132:359–372.
25. Arici C, Cheah WK, Ituarte PH, Morita E, Lynch TC, Siperstein AE, Duh QY, Clark OH. Can localization studies be used to direct focused parathyroid operations? Surgery. 2001. 129:720–729.
26. Haber RS, Kim CK, Inabnet WB. Ultrasonography for preoperative localization of enlarged parathyroid glands in primary hyperparathyroidism: comparison with (99m)technetium sestamibi scintigraphy. Clin Endocrinol (Oxf). 2002. 57:241–249.
27. Gawande AA, Monchik JM, Abbruzzese TA, Iannuccilli JD, Ibrahim SI, Moore FD Jr. Reassessment of parathyroid hormone monitoring during parathyroidectomy for primary hyperparathyroidism after 2 preoperative localization studies. Arch Surg. 2006. 141:381–384.
28. Mihai R, Palazzo FF, Gleeson FV, Sadler GP. Minimally invasive parathyroidectomy without intraoperative parathyroid hormone monitoring in patients with primary hyperparathyroidism. Br J Surg. 2007. 94:42–47.
29. Lew JI, Solorzano CC, Montano RE, Carneiro-Pla DM, Irvin GL 3rd. Role of intraoperative parathormone monitoring during parathyroidectomy in patients with discordant localization studies. Surgery. 2008. 144:299–306.
30. Rodgers SE, Hunter GJ, Hamberg LM, Schellingerhout D, Doherty DB, Ayers GD, Shapiro SE, Edeiken BS, Truong MT, Evans DB, Lee JE, Perrier ND. Improved preoperative planning for directed parathyroidectomy with 4-dimensional computed tomography. Surgery. 2006. 140:932–940.
31. Irvin GL 3rd, Prudhomme DL, Deriso GT, Sfakianakis G, Chandarlapaty SK. A new approach to parathyroidectomy. Ann Surg. 1994. 219:574–579.
32. Irvin GL 3rd, Sfakianakis G, Yeung L, Deriso GT, Fishman LM, Molinari AS, Foss JN. Ambulatory parathyroidectomy for primary hyperparathyroidism. Arch Surg. 1996. 131:1074–1078.
33. Carneiro DM, Solorzano CC, Nader MC, Ramirez M, Irvin GL 3rd. Comparison of intraoperative iPTH assay (QPTH) criteria in guiding parathyroidectomy: which criterion is the most accurate? Surgery. 2003. 134:973–979.
34. Clerici T, Brandle M, Lange J, Doherty GM, Gauger PG. Impact of intraoperative parathyroid hormone monitoring on the prediction of multiglandular parathyroid disease. World J Surg. 2004. 28:187–192.
35. Chiu B, Sturgeon C, Angelos P. Which intraoperative parathyroid hormone assay criterion best predicts operative success? A study of 352 consecutive patients. Arch Surg. 2006. 141:483–487.
36. Carneiro-Pla DM, Solorzano CC, Lew JI, Irvin GL 3rd. Long-term outcome of patients with intraoperative parathyroid level remaining above the normal range during parathyroidectomy. Surgery. 2008. 144:989–993.
37. Taylor J, Fraser W, Banaszkiewicz P, Drury P, Atkins P. Lateralization of parathyroid adenomas by intra-operative parathormone estimation. J R Coll Surg Edinb. 1996. 41:174–177.
38. Ito F, Sippel R, Lederman J, Chen H. The utility of intraoperative bilateral internal jugular venous sampling with rapid parathyroid hormone testing. Ann Surg. 2007. 245:959–963.
39. Boggs JE, Irvin GL 3rd, Carneiro DM, Molinari AS. The evolution of parathyroidectomy failures. Surgery. 1999. 126:998–1002.
40. Chen H, Pruhs Z, Starling JR, Mack E. Intraoperative parathyroid hormone testing improves cure rates in patients undergoing minimally invasive parathyroidectomy. Surgery. 2005. 138:583–587.
41. Irvin GL 3rd, Molinari AS, Figueroa C, Carneiro DM. Improved success rate in reoperative parathyroidectomy with intraoperative PTH assay. Ann Surg. 1999. 229:874–878.
42. Udelsman R. Six hundred fifty-six consecutive explorations for primary hyperparathyroidism. Ann Surg. 2002. 235:665–670.
43. Westerdahl J, Lindblom P, Bergenfelz A. Measurement of intraoperative parathyroid hormone predicts long-term operative success. Arch Surg. 2002. 137:186–190.
44. Irvin GL 3rd, Carneiro DM, Solorzano CC. Progress in the operative management of sporadic primary hyperparathyroidism over 34 years. Ann Surg. 2004. 239:704–708.
45. Grant CS, Thompson G, Farley D, van Heerden J. Primary hyperparathyroidism surgical management since the introduction of minimally invasive parathyroidectomy: Mayo Clinic experience. Arch Surg. 2005. 140:472–478.
46. Westerdahl J, Bergenfelz A. Unilateral versus bilateral neck exploration for primary hyperparathyroidism: five-year follow-up of a randomized controlled trial. Ann Surg. 2007. 246:976–980.
47. Siperstein A, Berber E, Barbosa GF, Tsinberg M, Greene AB, Mitchell J, Milas M. Predicting the success of limited exploration for primary hyperparathyroidism using ultrasound, sestamibi, and intraoperative parathyroid hormone: analysis of 1158 cases. Ann Surg. 2008. 248:420–428.
48. Sidhu S, Neill AK, Russell CF. Long-term outcome of unilateral parathyroid exploration for primary hyperparathyroidism due to presumed solitary adenoma. World J Surg. 2003. 27:339–342.
49. Lew JI, Irvin GL 3rd. Focused parathyroidectomy guided by intra-operative parathormone monitoring does not miss multiglandular disease in patients with sporadic primary hyperparathyroidism: a 10-year outcome. Surgery. 2009. 146:1021–1027.
50. Mun HC, Conigrave A, Wilkinson M, Delbridge L. Surgery for hyperparathyroidism: does morphology or function matter most? Surgery. 2005. 138:1111–1120.
51. Elliott DD, Monroe DP, Perrier ND. Parathyroid histopathology: is it of any value today? J Am Coll Surg. 2006. 203:758–765.
52. Carneiro-Pla DM, Romaguera R, Nadji M, Lew JI, Solorzano CC, Irvin GL 3rd. Does histopathology predict parathyroid hypersecretion and influence correctly the extent of parathyroidectomy in patients with sporadic primary hyperparathyroidism? Surgery. 2007. 142:930–935.
53. Kaplan EL, Yashiro T, Salti G. Primary hyperparathyroidism in the 1990s. Choice of surgical procedures for this disease. Ann Surg. 1992. 215:300–317.
54. Allendorf J, DiGorgi M, Spanknebel K, Inabnet W, Chabot J, Logerfo P. 1112 consecutive bilateral neck explorations for primary hyperparathyroidism. World J Surg. 2007. 31:2075–2080.
55. Duh QY. Presidential Address: Minimally invasive endocrine surgery--standard of treatment or hype? Surgery. 2003. 134:849–857.
56. Chen H, Mack E, Starling JR. Radioguided parathyroidectomy is equally effective for both adenomatous and hyperplastic glands. Ann Surg. 2003. 238:332–337.
57. Chen H, Mack E, Starling JR. A comprehensive evaluation of perioperative adjuncts during minimally invasive parathyroidectomy: which is most reliable? Ann Surg. 2005. 242:375–380.
58. Inabnet WB 3rd, Dakin GF, Haber RS, Rubino F, Diamond EJ, Gagner M. Targeted parathyroidectomy in the era of intraoperative parathormone monitoring. World J Surg. 2002. 26:921–925.
59. Jaskowiak NT, Sugg SL, Helke J, Koka MR, Kaplan EL. Pitfalls of intraoperative quick parathyroid hormone monitoring and gamma probe localization in surgery for primary hyperparathyroidism. Arch Surg. 2002. 137:659–668.




 PDF
PDF ePub
ePub Citation
Citation Print
Print



 XML Download
XML Download