Abstract
Background
Non-alcoholic fatty liver disease (NAFLD) is increasingly recognized as a major cause of liver-related morbidity and mortality. The underlying mechanisms of disease progression remain poorly understood, and primary therapy of NAFLD is not yet established. We investigated the effects of dietary oleate on the development and progression of NAFLD in a methionine- and choline-deficient (MCD) diet-fed animal model.
Methods
A total of 30 C57BL/6J mice were randomly divided into three groups (n=10 in each group) and fed various experimental diets for four weeks: chow, MCD diet, or OMCD (MCD diet with oleate, 0.5 mg/g/day). Liver samples were examined for steatohepatitis and fibrosis parameters and associated genes.
Results
Additional dietary oleate dramatically reduced MCD diet-induced hepatic steatosis. Hepatic carbohydrate responsive element-binding protein was overexpressed in MCD diet-fed mice, and dietary oleate prevented this overexpression (P<0.001). Dietary oleate partially prevented MCD diet-induced serum level increases in aspartate aminotransferase and alanine aminotransferase (P<0.001, respectively). The mRNA expressions of hepatic monocyte chemoattractant protein 1, tumor necrosis factor-α and matrix metalloproteinase-9 were increased in MCD diet-fed mice, and this overexpression of inflammatory molecules was prevented by dietary oleate (P<0.001). Hepatic pericellular fibrosis was observed in MCD diet-fed mice, and dietary oleate prevented this fibrosis. Altogether, dietary oleate prevented MCD diet-induced hepatic steatosis, inflammation and fibrosis.
Non-alcoholic fatty liver disease (NAFLD) represents a spectrum of disorders characterized by hepatic fat accumulation ranging from simple steatosis to severe non-alcoholic steatohepatitis (NASH) with centrilobular necroinflammation and has been increasingly recognized as a major cause of liver-related morbidity and mortality [1]. Although the underlying mechanisms of disease progression remain poorly understood, insulin resistance, oxidative stress, and an inflammatory cascade are believed to play important roles in the development and progression of this disease [1-3]. The treatment of NAFLD consists of modification of underlying risk factors and detection of patients with liver cirrhosis. Although some agents provide modest improvements in liver function tests and histologic parameters, primary therapy for NAFLD has not yet been established [2].
The methionine- and choline-deficient (MCD) diet is a well-established and widely-used nutritional model of human NAFLD. This high sucrose-containing diet lacks methionine and choline in its composition, contains a higher lipid composition than a normal chow diet, and causes hepatic steatosis and inflammation that mimics NAFLD in humans [4,5]. Although the mechanisms responsible for the development of hepatic steatosis due to the MCD diet are not fully understood, recent studies suggest that the MCD diet increases hepatic fatty acid uptake and decreases very low-density lipoprotein (VLDL) secretion [4].
Oleic acid (18:1n-9), the richest source of fatty acid in olive oil, is a monounsaturated fatty acid (MUFA). Recently, dietary olive oil was reported to decrease hepatic lipid content in rats fed an MCD diet [6]. Considering that only 15% of fatty acid stored in the liver is of dietary origin in NAFLD patients [7], reduced plasma free fatty acid (FFA) and de novo lipogenesis were suggested as possible mechanisms to prevent the accumulation of fatty acids in the liver under an olive oil-enriched diet [6]. In fact, MUFA from safflower oil was reported to decrease lipogenesis in the rat liver [8]. Additionally, recent studies show that a diet rich in olive oil decreases hepatic injury and apoptosis in mice fed an MCD diet [9]. However, to our knowledge, there are few studies that have investigated the effect of dietary MUFA on each stage of NAFLD development and progression.
In this study, we investigated the effect of dietary oleate on the development and progression of NAFLD in a MCD-diet mouse model. This study presents some clues to understanding the mechanisms through which the MCD diet induces steatohepatitis and describes how dietary MUFA prevents steatohepatitis in this animal model. As a result, this study suggests dietary oleate as a nutritional option for NAFLD prevention and treatment.
Eight-week-old C57BL/6J mice (Samtako Inc., Osan, Korea) weighing 20 to 21 g were maintained at ambient temperature (22±1℃) on 12-hour light-dark cycles with free access to water and diet. Initially, all mice were fed a chow diet during a one-week quarantine and acclimation period. Then, at nine weeks of age, mice displaying no abnormal findings at the end of the quarantine and acclimation period were randomly divided into three groups. The mice in the chow group (n=10) were fed a normal chow diet; the mice in the MCD group (n=10) were fed an MCD diet (Dyets Inc., Bethlehem, PA, USA); the mice in the OMCD group (n=10) were fed an MCD diet with oleate supplementation (0.5 mg/g/day; Sigma Aldrich, St. Louis, MO, USA) throughout the four weeks of the experimental period. Oleate was orally administrated with a 10 cc syringe, and body weights were measured every other day. Mice were sacrificed at 13 weeks of age. The animals were euthanized at the end of a dark cycle after overnight fasting for tissue sampling. Blood was collected by cardiac puncture, and the livers were isolated, immediately freeze-clamped in liquid nitrogen, and stored at -80℃ until analysis. All experimental procedures were performed under sterile conditions and were approved by the Institutional Animal Care and Use Committee at Yonsei University College of Medicine.
Plasma levels of aspartate aminotransferase (AST) and alanine aminotransferase (ALT) were assayed by routine automated laboratory methods.
Livers were weighed to allow the calculation of relative liver weight (percentage of liver weight to body weight). Livers were fixed in 10% buffered formalin, embedded in paraffin, and sectioned at 3 µm. Standard hematoxylin and osin (H&E) and trichrome staining was performed [10]. Fresh tissue was frozen immediately after each animal was dissected, and the tissue was placed in pre-labeled base molds filled with embedding medium used for frozen tissue to ensure the optimal cutting temperature (OCT). Routine sections were cut at 7 µm, frozen and stained with Oil-red O. For the evaluation of hepatic steatosis and fibrosis, the average areas (%) of the fat droplets and fibrosis within hepatocytes were measured with the aid of an image analyzer in three randomly selected fields (magnification, ×100) of each section stained with Oil-red O and trichrome [11].
Immunohistochemistry was performed with an antibody to carbohydrate responsive element-binding protein (ChREBP) (Thermo Fisher Scientific Inc., Rockford, IL, USA) on paraffin-embedded sections. Immunohistochemical staining was performed with an ABC staining system (Santa Cruz Biotechnology, Santa Cruz, CA, USA) [12].
Total RNA was isolated from mouse liver tissue using Trizol reagent (Invitrogen, Carlsbad, CA, USA) and quantified using a Nano Drop instrument (ND-1000; DM Science, Seoul, Korea). Following RNA extraction, 4 µL RNA was treated with 1 U DNase I to remove all contaminating genomic DNA. DNase-treated RNA was subsequently used for cDNA synthesis using MMLV reverse transcriptase (Promega, Madison, WI, USA): 1 µL oligo dT primer was added to 4 µL RNA in 5× MMLV reaction buffer, 2.5 mM each dNTP, 1 U RNasin ribonuclease inhibitor and MMLV reverse transcriptase (200 units). cDNA was stored at -20℃.
Real-time quantitative reverse transcription-polymerase chain reaction (RT-PCR) analysis was performed with an ABI 7500 instrument and software (Applied Biosystems, Foster City, CA, USA). PCR reactions were performed in triplicate in a final volume of 20 µL according to the manufacturer's protocol. Mouse ChREBP mRNA expression was analyzed using Taqman probes (Mm02342723_m1; Applied Biosystems). For each assay, a standard curve was obtained by analyzing a dilution series of pooled cDNA samples for the relevant gene. Data were analyzed with Sequence Detector 1.7 software (Applied Biosystems). Mouse glyceraldehyde-3-phosphate dehydrogenase (GAPDH) was used as the internal standard to control for variability, and results were expressed as a ratio of the gene expression relative to that of GAPDH.
The hepatic expressions of the mRNAs for apolipoprotein B100 (APOB100), monocyte chemoattractant protein 1 (MCP1), tumor necrosis factor-α (TNF-α) and matrix metalloproteinase-9 (MMP-9) were assessed by semi-quantitative RT-PCR analysis using GAPDH as an internal control gene. RT-PCR products were electrophoresed on a 1% (w/v) agarose gel, the gel was stained with ethidium bromide, and bands were visualized by UV light. The primers used for RT-PCR are listed in Table 1. Samples from all experimental animals were used for this analysis.
All statistical analyses were performed with SPSS software version 15.0 (SPSS Inc., Chicago, IL, USA). Values were expressed as mean±standard deviation. Statistical analyses were performed using the unpaired Student's t-test or one-way ANOVA. Data with a P value less than 0.05 were considered statistically significant.
After the four week-experimental period, the body weights of the MCD diet-fed mice significantly decreased compared to those of the chow diet-fed mice (chow vs. MCD vs. OMCD, 25.0±0.7 g vs. 15.3±0.8 g vs. 15.6±1.2 g, P<0.001) (Table 2). Relative liver weights in the MCD diet-fed groups were significantly increased compared to those in the chow diet-fed group. Between the MCD diet-fed groups, the increase in relative liver weight in mice fed an MCD diet only was more prominent than that in mice fed an MCD diet with oleate (chow vs. MCD vs. OMCD, 4.8±0.5% vs. 6.5±0.4% vs. 5.8±0.6%, P<0.001) (Table 2).
To investigate and compare the extent of hepatic steatosis among the groups, Oil-Red-O staining of mice liver samples was performed. In the MCD diet-fed mice, numerous fat droplets were observed within hepatocytes, and the total fat droplet area was markedly increased in the livers of MCD diet-fed mice (chow vs. MCD, 0.05±0.01% vs. 14.31±0.35%, P<0.001) (Fig. 1). This increase in hepatic lipid droplets in mice fed an MCD diet was attenuated by dietary oleate. In the livers of oleate-treated MCD diet-fed mice, lipid droplets were significantly decreased compared to those of the MCD diet-fed mice (MCD vs. OMCD, 14.31±0.35% vs. 3.65±0.04%, P<0.001) (Fig. 1).
To investigate the effect of dietary MUFA on hepatic de novo lipogenesis, ChREBP expression in each group was evaluated. Immunohistochemical staining revealed that hepatic ChREBP was overexpressed in mice fed an MCD diet compared to that of chow diet-fed mice (Fig. 2A and B). Interestingly, dietary oleate completely attenuated MCD diet-induced overexpression of hepatic ChREBP (Fig. 2C). The results of quantitative RT-PCR demonstrate that the mRNA expression of hepatic ChREBP was markedly increased by the MCD diet, and oleate intake prevented this increase (chow vs. MCD vs. OMCD, 1.00±0.07 AU vs. 1.90±0.14 AU vs. 1.10±0.07 AU, P<0.001) (Fig. 2D).
Hepatic APOB100 expression was evaluated to investigate the effect of dietary MUFA on hepatic VLDL excretion. The mRNA expression of hepatic APOB100 tended to be reduced in the MCD diet-fed mice, and dietary oleate prevented this reduction (chow vs. MCD vs. OMCD, 1.00±0.25 AU vs. 0.75±0.25 AU vs. 1.10±0.39 AU, P=0.065) (Fig. 2E).
To investigate the hepatic inflammatory status of each group, serum AST and ALT levels were measured. As expected, the MCD diet increased serum AST and ALT levels (AST, chow vs. MCD, 85.3±5.9 IU/L vs. 465.5±6.8 IU/L, P<0.001; ALT, chow vs. MCD, 38.7±9.7 IU/L vs. 658.3±8.4 IU/L, P<0.001) (Table 2). Oleate intake partially prevented these increases in serum AST and ALT levels (AST, MCD vs. OMCD, 465.5±6.8 IU/L vs. 356.5±6.4 IU/L, P<0.001; ALT, MCD vs. OMCD, 658.3±8.4 IU/L vs. 450.2±7.2 IU/L, P<0.001) (Table 2). H&E staining of mice liver samples displayed increased inflammatory cells and focal aggregates of inflammatory cells in MCD diet-fed mice compared to the responses in chow diet-fed or OMCD diet-fed mice (Fig. 3). The results of semi-quantitative RT-PCR demonstrate that the mRNA expressions of hepatic MCP1, TNF-α and MMP-9 were increased in the MCD diet-fed mice, and this overexpression of inflammatory cytokines was prevented by dietary oleate (MCP1, chow vs. MCD vs. OMCD, 1.00±0.07 AU vs. 1.95±0.14 AU vs. 1.51±0.11 AU, P<0.001; TNF-α, 1.00±0.08 AU vs. 1.93±0.11 AU vs. 1.12±0.11 AU, P<0.001; MMP-9, 1.00±0.07 AU vs. 1.83±0.11 AU vs. 1.24±0.12 AU, P<0.001) (Fig. 4).
Trichrome staining of mice liver samples was used to evaluate the hepatic pericellular fibrosis in each group. Pericellular fibrosis and numerous collagen fibers were observed in livers from the MCD diet-fed mice, but the number of liver collagen fibers was not higher than that of chow diet-fed mice in oleate-treated mice (relative fibrosis area, chow vs. MCD vs. OMCD, 1.00±0.05 AU vs. 1.96±0.06 AU vs. 1.10±0.05 AU, P<0.001) (Fig. 5).
This study demonstrated that dietary oleate prevented MCD diet-induced steatohepatitis and hepatic fibrosis via an increase in hepatic triglyceride excretion and decreases in hepatic de novo lipogenesis and inflammation. The MCD diet is a well-established and widely-used nutritional model of steatohepatitis thatcauses hepatic steatosis and inflammation that mimics NAFLD in humans [4,5]. In this study, as expected, hepatic steatosis developed in the MCD diet-fed mice. Additionally, the MCD diet tended to reduce hepatic APOB100. This result suggests a decrease in hepatic VLDL excretion and is consistent with previous studies [4,13]. Surprisingly, our results showed that the MCD diet induced an important lipogenic transcription factor in the liver, ChREBP. ChREBP regulates hepatic de novo lipogenesis in response to elevated glucose concentrations by binding lipogenic enzyme genes [14,15]. Previous studies reported the opposite results for another lipogenic transcription factor, sterol regulatory element-binding protein-1 (SREBP-1) [4,16,17]. In those studies, reduced mRNA expression or nuclear levels of hepatic SREBP-1 were observed in the MCD diet-fed mice compared with those in the chow diet-fed mice, but no consistent reduction in fatty acid synthesis genes, such as acetyl-CoA carboxylase (ACC) and fatty acid synthase (FAS), was noted [16,17]. Our results might explain this discrepancy; ACC and FAS are regulated not only by SREBP-1 but also by ChREBP; thus, the overexpression of ChREBP due to an MCD diet might maintain the expression of fatty acid synthesis genes. In fact, hepatic de novo lipogenesis is not known to contribute to the development of hepatic steatosis in the MCD diet animal model [4,13,16,17]. Although the MCD diet induced hepatic ChREBP overexpression in our study, this result does not mean that hepatic steatosis is due to increased hepatic lipogenesis because MCD diet-induced hepatic ChREBP overexpression could be a compensatory response for decreased hepatic de novo lipogenesis. Furthermore, we did not investigate downstream lipogenic regulation of ChREBP. Nevertheless, considering glucose-induced ChREBP activation and the up-regulation of ChREBP in a diabetic condition, the steatogenetic potential of this overexpression of hepatic ChREBP by an MCD diet might be magnified in the condition of hyperglycemia, even if the increase in hepatic de novo lipogenesis is not a main mechanism of MCD diet-induced hepatic steatosis. Actually, in Otsuka Long-Evans Tokushima Fatty rats, an animal model of type 2 diabetes with insulin resistance and hyperglycemia, it was reported that the development of hepatic fat accumulation, inflammation and fibrosis due to an MCD diet was accelerated compared with that in non-diabetic control rats [18]. In a report with db/db mice, another diabetic animal model, hepatic fibrosis induced by an MCD diet was more prominent than that in non-diabetic db/m mice [19].
Previous studies have reported the effect of dietary MUFA against steatohepatitis in NAFLD animal models. A study with rats fed an MCD diet show that the extent of hepatic fatty infiltration and hepatic triglyceride content were lower in the rats fed an MCD diet with olive oil (0.45 mg/g rat weight) than in the rats fed an MCD diet only [6]. The authors of that study suggested MUFA from olive oil might inhibit hepatic triglyceride synthesis on the basis of prior studies reporting reduced lipogenesis in the rat liver due to dietary MUFA from safflower oil [6,8]. In the present study, hepatic steatosis was reduced in mice fed an MCD diet with oleate compared with that in the mice fed an MCD diet only. Additionally, dietary oleate almost completely prevented the decrease in hepatic APOB100 expression, and the MCD diet induced overexpression of hepatic ChREBP in mice. These results suggest that dietary oleate prevents hepatic steatosis by maintaining hepatic VLDL excretion, as well as by reducing hepatic de novo lipogenesis in this animal model. As mentioned above, increased hepatic ChREBP does not mean that hepatic de novo lipogenesis contributes to the development of steatohepatitis in this animal model. However, we can suggest that dietary oleate prevented MCD diet-induced hepatic steatosis, and this effect, at least in part, is associated with the reduced expression of hepatic ChREBP and suppression of hepatic de novo lipogenesis. In addition, our results also suggest that dietary oleate might be more effective against hepatic steatosis in a hyperglycemic or insulin-resistant status.
In the present study, we cannot confirm whether the change in hepatic ChREBP expression is a direct effect of the MCD diet or dietary oleate. Although it has been reported that polyunsaturated fatty acids (PUFA) suppress ChREBP activity in primary hepatocytes by increasing ChREBP mRNA decay [20], and high glucose concentration down-regulates the expression of ChREBP mRNA in insulinoma cells [21], the regulation of ChREBP mRNA transcription or translation is not well understood [14]. The mechanisms through which the MCD diet increases hepatic ChREBP expression and dietary oleate prevents this overexpression need to be further investigated.
H&E staining of the liver showed increased and focally aggregated inflammatory cells in the livers of MCD diet-fed mice. Dietary oleate prevented this aggregation of hepatic inflammatory cells. These results are consistent with our results of increased MCP1 mRNA expression in the liver of MCD diet-fed mice and reduced expression by dietary oleate. MCP-1 is a potent chemotactic factor for monocytes and is derived predominantly from macrophages and endothelial cells [22,23]. The MCD diet and dietary oleate also affected the expressions of other hepatic inflammatory molecules, such as TNF-α and MMP-9. Biologically, TNF-α activates a cascade of cytokine production [24], and MMP-9 plays a crucial role for the migration, extravasation, and infiltration of immune cells, including monocytes [25]. TNF-α, in particular, is implicated in the pathogenesis of NAFLD [2]. Elevated levels of TNF-α have been detected in obese patients with insulin resistance and also in patients with NASH [2]. In our results, the MCD diet induced TNF-α and MMP-9 mRNA expressions in the liver, and dietary oleate prevented the up-regulation of these inflammatory molecules. To our knowledge, this is the first study to report the effects of dietary MUFA on hepatic inflammatory molecules in a NAFLD animal model.
To explain the pathogenesis of NAFLD, a "multi-hit" (formerly "double-hit") hypothesis has been widely accepted; insulin resistance and hepatic steatosis - the first hit - and subsequent development of inflammation or fibrosis - the second hit [2]. With trichrome staining, it was demonstrated that dietary oleate prevented MCD diet-induced hepatic fibrosis. This result suggests that dietary MUFA might prevent the development of cirrhosis resulting from NAFLD. Further, considering the "multi-hit hypothesis," our results showing the effects of dietary oleate on hepatic steatosis, inflammation and fibrosis suggest that dietary MUFA has beneficial effects in every step of NAFLD development and progression.
In conclusion, our data suggest that dietary MUFA can prevent hepatic steatosis by reducing hepatic ChREBP overexpression, as well as by inducing APOB100 expression. Additionally, our data suggest that dietary MUFA inhibits the development of hepatic inflammation and fibrosis by suppressing several inflammatory molecules. Altogether, dietary oleate has beneficial effects in every step of the development and progression of NAFLD and may be a nutritional option for NAFLD prevention and treatment.
Figures and Tables
Fig. 1
The effects of the methionine- and choline-deficient diet (MCD) diet and dietary oleate on hepatic fat accumulation. Oil-red-O staining of frozen liver sections. Scale bars, 200 µm. (A) Chow diet-fed mice. (B) MCD diet-fed mice. (C) MCD diet with oleate (0.5 mg/g/day for 4 weeks)-fed mice. (D) The areas of fat droplets, measured by image analyzer in three randomly selected fields (magnification, ×100) of each liver section. Chow, normal chow diet; OMCD, MCD diet with oleate. aP<0.05 vs. chow, bP<vs. MCD.
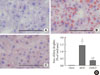
Fig. 2
The effects of the methionine- and choline-deficient diet (MCD) diet and dietary oleate on hepatic carbohydrate responsive element-binding protein (ChREBP) and apolipoprotein B (apo B) expression. Immunohistochemical detection of ChREBP in liver paraffin-embedded sections. Scale bars, 50 µm. (A) Chow diet-fed mice. (B) MCD diet-fed mice. (C) MCD diet with oleate (0.5 mg/g/day for 4 weeks)-fed mice. (D) Real-time reverse transcription-polymerase chain reaction (RT-PCR) quantification of ChREBP mRNA in mouse liver samples from each group. (E) Real-time RT-PCR quantification of APOB100 mRNA in mouse liver samples from each group. AU, arbitrary unit; Chow, normal chow diet; OMCD, MCD diet with oleate. aP<0.05 vs. chow, bP<vs. MCD.
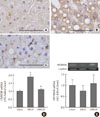
Fig. 3
Liver histology in mice fed each experimental diet. H&E staining of liver paraffin-embedded sections. Scale bars, 200 µm. (A) Chow diet-fed mice. (B) MCD diet-fed mice. (C) MCD diet with oleate (0.5 mg/g/day for 4 weeks)-fed mice. Arrows indicate the focal aggregation of inflammatory cells. Chow, normal chow diet; MCD, methionine- and choline-deficient diet; OMCD, MCD diet with oleate.
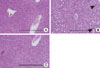
Fig. 4
The effects of the methionine- and choline-deficient diet (MCD) diet and dietary oleate on hepatic inflammatory molecules. Semi-quantitative reverse transcription-polymerase chain reaction (RT-PCR) for inflammatory molecules in mouse liver samples from each group. (A) MCP1. (B) TNF-α. (C) MMP-9. MCP1, monocyte chemotactic protein 1; TNF-α, tumor necrosis factor-α; MMP-9, matrix metalloproteinase-9; GAPDH, glyceraldehyde-3-phosphate dehydrogenase; AU, arbitrary unit; Chow, normal chow diet; MCD, methionine- and choline-deficient diet; OMCD, MCD diet with oleate (0.5 mg/g/day for 4 weeks). aP<0.05 vs. chow, bP<vs. MCD.
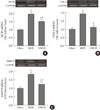
Fig. 5
The effects of the methionine- and choline-deficient diet (MCD) diet and dietary oleate on hepatic fibrosis. Trichrome staining of liver paraffin-embedded sections. Scale bars, 200 µm. (A) Chow diet-fed mice. (B) MCD diet-fed mice. (C) MCD diet with oleate (0.5 mg/g/day for 4 weeks)-fed mice. (D) The areas of fibrosis, measured by image analyzer in three randomly selected fields (magnification, ×100) of each liver section. AU, arbitrary unit; Chow, normal chow diet; OMCD, MCD diet with oleate. aP<0.05 vs. chow, bP<vs. MCD.
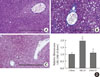
Table 2
Clinical characteristics of mice after four weeks of experimental diets

Values represent mean±standard deviation for n=10 in each group.
Chow, normal chow diet; MCD, methionine- and choline-deficient diet; OMCD, MCD diet with oleate (0.5 mg/g/day); AST, aspartate aminotransferase; ALT, alanine aminotransferase.
aP<0.05 for MCDD vs. chow, bP<0.05 for OMCDD vs. chow, cP<0.05 for OMCD vs. MCD.
ACKNOWLEDGMENTS
This work was supported by the Korea Science and Engineering Foundation (KOSEF) grant funded by the Korean government (MEST) (grant number, 2010-0028367).
References
1. Farrell GC, Larter CZ. Nonalcoholic fatty liver disease: from steatosis to cirrhosis. Hepatology. 2006. 43:2 Suppl 1. S99–S112.
2. Lewis JR, Mohanty SR. Nonalcoholic fatty liver disease: a review and update. Dig Dis Sci. 2010. 55:560–578.
3. Cheung O, Sanyal AJ. Recent advances in nonalcoholic fatty liver disease. Curr Opin Gastroenterol. 2009. 25:230–237.
4. Rinella ME, Elias MS, Smolak RR, Fu T, Borensztajn J, Green RM. Mechanisms of hepatic steatosis in mice fed a lipogenic methionine choline-deficient diet. J Lipid Res. 2008. 49:1068–1076.
5. Schattenberg JM, Singh R, Wang Y, Lefkowitch JH, Rigoli RM, Scherer PE, Czaja MJ. JNK1 but not JNK2 promotes the development of steatohepatitis in mice. Hepatology. 2006. 43:163–172.
6. Hussein O, Grosovski M, Lasri E, Svalb S, Ravid U, Assy N. Monounsaturated fat decreases hepatic lipid content in non-alcoholic fatty liver disease in rats. World J Gastroenterol. 2007. 13:361–368.
7. Donnelly KL, Smith CI, Schwarzenberg SJ, Jessurun J, Boldt MD, Parks EJ. Sources of fatty acids stored in liver and secreted via lipoproteins in patients with nonalcoholic fatty liver disease. J Clin Invest. 2005. 115:1343–1351.
8. Wong SH, Nestel PJ, Trimble RP, Storer GB, Illman RJ, Topping DL. The adaptive effects of dietary fish and safflower oil on lipid and lipoprotein metabolism in perfused rat liver. Biochim Biophys Acta. 1984. 792:103–109.
9. Li ZZ, Berk M, McIntyre TM, Feldstein AE. Hepatic lipid partitioning and liver damage in nonalcoholic fatty liver disease: role of stearoyl-CoA desaturase. J Biol Chem. 2009. 284:5637–5644.
10. Abiru S, Migita K, Maeda Y, Daikoku M, Ito M, Ohata K, Nagaoka S, Matsumoto T, Takii Y, Kusumoto K, Nakamura M, Komori A, Yano K, Yatsuhashi H, Eguchi K, Ishibashi H. Serum cytokine and soluble cytokine receptor levels in patients with non-alcoholic steatohepatitis. Liver Int. 2006. 26:39–45.
11. Oz HS, Im HJ, Chen TS, de Villiers WJ, McClain CJ. Glutathione-enhancing agents protect against steatohepatitis in a dietary model. J Biochem Mol Toxicol. 2006. 20:39–47.
12. Tomita K, Tamiya G, Ando S, Ohsumi K, Chiyo T, Mizutani A, Kitamura N, Toda K, Kaneko T, Horie Y, Han JY, Kato S, Shimoda M, Oike Y, Tomizawa M, Makino S, Ohkura T, Saito H, Kumagai N, Nagata H, Ishii H, Hibi T. Tumour necrosis factor alpha signalling through activation of Kupffer cells plays an essential role in liver fibrosis of non-alcoholic steatohepatitis in mice. Gut. 2006. 55:415–424.
13. Yao ZM, Vance DE. The active synthesis of phosphatidylcholine is required for very low density lipoprotein secretion from rat hepatocytes. J Biol Chem. 1988. 263:2998–3004.
14. Uyeda K, Repa JJ. Carbohydrate response element binding protein, ChREBP, a transcription factor coupling hepatic glucose utilization and lipid synthesis. Cell Metab. 2006. 4:107–110.
15. Stoeckman AK, Ma L, Towle HC. Mlx is the functional heteromeric partner of the carbohydrate response element-binding protein in glucose regulation of lipogenic enzyme genes. J Biol Chem. 2004. 279:15662–15669.
16. Rizki G, Arnaboldi L, Gabrielli B, Yan J, Lee GS, Ng RK, Turner SM, Badger TM, Pitas RE, Maher JJ. Mice fed a lipogenic methionine-choline-deficient diet develop hypermetabolism coincident with hepatic suppression of SCD-1. J Lipid Res. 2006. 47:2280–2290.
17. Larter CZ, Yeh MM, Haigh WG, Williams J, Brown S, Bell-Anderson KS, Lee SP, Farrell GC. Hepatic free fatty acids accumulate in experimental steatohepatitis: role of adaptive pathways. J Hepatol. 2008. 48:638–647.
18. Ota T, Takamura T, Kurita S, Matsuzawa N, Kita Y, Uno M, Akahori H, Misu H, Sakurai M, Zen Y, Nakanuma Y, Kaneko S. Insulin resistance accelerates a dietary rat model of nonalcoholic steatohepatitis. Gastroenterology. 2007. 132:282–293.
19. Sahai A, Malladi P, Pan X, Paul R, Melin-Aldana H, Green RM, Whitington PF. Obese and diabetic db/db mice develop marked liver fibrosis in a model of nonalcoholic steatohepatitis: role of short-form leptin receptors and osteopontin. Am J Physiol Gastrointest Liver Physiol. 2004. 287:G1035–G1043.
20. Dentin R, Benhamed F, Pegorier JP, Foufelle F, Viollet B, Vaulont S, Girard J, Postic C. Polyunsaturated fatty acids suppress glycolytic and lipogenic genes through the inhibition of ChREBP nuclear protein translocation. J Clin Invest. 2005. 115:2843–2854.
21. Wang H, Wollheim CB. ChREBP rather than USF2 regulates glucose stimulation of endogenous L-pyruvate kinase expression in insulin-secreting cells. J Biol Chem. 2002. 277:32746–32752.
22. Matsushima K, Larsen CG, DuBois GC, Oppenheim JJ. Purification and characterization of a novel monocyte chemotactic and activating factor produced by a human myelomonocytic cell line. J Exp Med. 1989. 169:1485–1490.
23. Rollins BJ. Chemokines. Blood. 1997. 90:909–928.
24. Jiménez-Gómez Y, Lopez-Miranda J, Blanco-Colio LM, Marin C, Perez-Martinez P, Ruano J, Paniagua JA, Rodriguez F, Egido J, Perez-Jimenez F. Olive oil and walnut breakfasts reduce the postprandial inflammatory response in mononuclear cells compared with a butter breakfast in healthy men. Atherosclerosis. 2009. 204:e70–e76.
25. Sun J, Feng A, Zhang Y, Sun S, Hu W, Yang M, Wei F, Qu X. Fucoidan increases TNF-alpha-induced MMP-9 secretion in monocytic cell line U937. Inflamm Res. 2010. 59:271–276.




 PDF
PDF ePub
ePub Citation
Citation Print
Print



 XML Download
XML Download