INTRODUCTION
Standardization of the technique, and specific experience and training with IONM device are essential for optimal use.
Correct functioning of the IONM equipment with regard to nerve stimulation “stimulation site” and the signal recording and reproducing “recording site.”
Intact and sufficient nerve conduction, including transfer into an adequate muscle action of the stimulated vocalis muscle.
Exclusion of pre-existing or endotracheal-induced non-nervous disorders of the vocal cord mobility.
STANDARDIZATION OF RLN MONITORING
Preoperative and postoperative laryngoscopy and intraoperative EMG are inseparable units. The preoperative intraoperative EMG findings cannot be assessed without preoperative laryngoscopy findings. Similarly, the postoperative intraoperative EMG findings are also not possible to correlate without the postoperative laryngoscopy findings interpretation. Thus, preoperative and postoperative laryngeal examinations are a prerequisite for the clinical use of the IONM method. A preoperative laryngeal detection of RLN palsy must be interpreted with caution, because, in spite of paralyzed vocal motility, a nerve can still present with a positive EMG signal. Therefore, for the IONM, the following explanations refer to the situation of a preoperatively intact vocal laryngeal mobility, but not to the situation of a pre-existing vocal cord paresis.
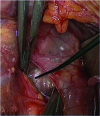
The routine intraoperative check of the functional status of the IONM system to prove the concordance of preoperative vocal cord movement and neurostimulation. The correctness evaluation method requires nerve stimulation outside the potential operative area of the RLN. For this, the ipsilateral vagal stimulation is the safest. The results of the largest evaluation study carried out for this purpose clearly demonstrated the importance of this approach, i.e., pre-excision vagal nerve (VN) stimulation (Fig. 1) (1523).
To achieve adequate nerve stimulation, the RLN and the VN should be stimulated beyond the threshold current of 0.3–0.8 mA. The optimal current strength for nerve stimulation is 1–2 mA. There is no further improvement and achievement of the muscle response of the vocalis muscle with a higher current strength (24).
Anesthesia management during intraoperative neuromonitoring does not require the use of muscle relaxants during the neuromonitoring phase, since these can impair the vocalis muscle response leading to intraoperative misinterpretations (25). During induction anesthesia, the use of short-acting muscle relaxants (succinylcholine [Anectine®; Sandoz Inc., Princeton, NJ, USA] 2.0–2.5 mg/kg or rocuronium [Zemuron™, Merck Canada Inc., Kirkland, Canada] or atracurium [Tracrium®; GlaxoSmithkline Pharmaceuticals Ltd., Mumbai, India] 0.5 mg/kg) is recommended in order to ensure adequate muscle activity when using nerve stimulation (25).
The correct IONM apparatus requires adequate placement of the cable connections, neutral electrodes, and EMG signal electrodes (i.e., needle electrodes or EMG endotracheal tube electrodes) (Fig. 2) (24). When the needle electrodes are used, they must be inserted into the vocalis muscle separately for each of the operated sides (1524). When EMG endotracheal tube electrodes are used, it is necessary to ensure that the cuff of the tube is located below the glottal plane and the signal derivation electrodes above, i.e., in the glottal plane of the vocal folds, and not rotated after the patient has been positioned (Fig. 3) (24). The proper contact of the EMG tube with the vocal cords is indicated by the following verification test: 1) impedance values readable on the IONM monitor less than 5 kΩ per electrode; 2) repeat laryngeal examination after the patient's head extension to check possible tube displacement; 3) respiratory variation; 4) tap test; and 5) intraoperative V1 above 500 mcV (Table 3) (24).
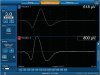
The unequivocal determination of a regular nerve conduction and consecutive muscle action of the ipsilateral vocalis muscle requires qualitative and quantitative EMG recordings with amplitude and latency by the IONM device, since the sole signal sound reproduction cannot reliably distinguish between an artifact signal and a regular muscular action potential (15). The use of modern audio and EMG signal equipment is recommended to enhance, if possible, the documentation of the stimulation EMG (Fig. 4) (15). Not only does this allow a reliable detection of the muscle potential but the stimulation of the RLN or the VN provides a latency of the recurrent nerve and the vagus nerve significantly different from one another due to the different nerve lengths (Table 4) (15). The different nerve lengths of the left and right nasal vagus also allow the respective EMG to be assigned to the right or left VN during stimulation of the vagus nerve for a secure lateral allocation (1524).
TROUBLESHOOTING AND CONSEQUENCES FOR INTRAOPERATIVE SIGNAL LOSS
Positive LT independent of the location of the stimulation: consider error of the “recording site,” i.e., EMG endotracheal tube dislocation or needle dislocation. This necessitates a correction of the tube or needle electrode position in case of the use of needle electrodes.
A normal EMG and/or positive LT during stimulation of the RLN at the point of entry into the larynx, but a lack of an EMG response or missing LT when stimulating the vagus nerve: consider a real loss of function of the ipsilateral RLN. In this type of nerve disorder, the location of the nerve lesion can usually be localized by “mapping” (type 1, point/segmental injury, Fig. 7).
A lack of an EMG response and a missing LT regardless of the location of the stimulation (RLN or vagus nerve) means a diffuse nervous disorder (type-2 global injury, Fig. 7), assuming correct stimulation and exclusion of the causes mentioned above.
If there is any uncertainty regarding the function of the equipment, tube position, or position of the needle electrode, the contralateral VN should be stimulated. When the nerve function of the contralateral VN is intact, a disturbed nerve function can be assumed in the absence of a stimulation response from the side in question.
CONCLUSIONS AND ASSESSMENTS IN CASE OF RLN INJURY
When intraoperative neuromonitoring is used, the following points should be considered: 1) compliance with the standards set-up (Table 2) and 2) systematic use of troubleshooting algorithms in the case of intraoperative signal loss (Fig. 5). Both points are essential for a correct interpretation of intraoperative EMG signals and, in the case of intraoperative signal loss, to draw appropriate conclusions.
Since the signal tone alone does not differ sufficiently reliably between artifact and regular muscle action potential, the simultaneous EMG recording alone represents the “gold standard” of the nerve function assessment. The only and best documentation currently available for an expert assessment of the intraoperative nerve function state is the EMG expression of ipsilateral vagal stimulation before and after resection.
The sensitivity of intraoperative neuromonitoring with regard to the assessment that postoperative RLN paresis follows an intraoperative signal failure is still unsatisfactory. Nevertheless, the intraoperative signal failure represents the best currently available technique, significantly superior risk criterion vs. the only anatomical-visual assessment for the prediction of a postoperative recurrence paresis. Thus, in case of a signal loss on the first operated side within an intended bilateral procedure the resection of the second side should be dispensed in the same session until the recovery of the nerve in order to avoid the risk of bilateral recurrent palsy. The reason for the premature bilateral resection in this situation has to be linked to high-threshold conditions, since there are very few indications under which the resection of the contralateral side could not occur at a later time.
Preoperative patient information (informed consent) should adequately take into account this neuromonitoring-dependent procedural strategy by informing the patient that intraoperative signal failure of the first operated side is generally dispensed with a 2-step procedure for the sake of avoiding 2-way RLN paresis more safely.




 PDF
PDF ePub
ePub Citation
Citation Print
Print











 XML Download
XML Download