Abstract
Spontaneous intracerbral hemorrhage (SICH) and chronic subdural hematoma (CSDH) represent the most common types of intracranial hematoma. However, SICH during the progression of CSDH is a very rare disease entity due to etiologic differences. The purpose of this report is to alert neurosurgeons to the existence of this condition and to consider possible mechanisms. An 87-year-old man presented with a sudden onset of headache, followed by quadriparesis. Brain computed tomography scan revealed CSDH in the left hemisphere and remote SICH in the right basal ganglia. CSDH was treated with burr hole drainage, however, the deep parenchymal hematoma was treated conservatively. The patient had a progressive recovery with a good outcome. We recently experienced a case of SICH that occurred during the progression of CSDH. To our knowledge, this is the first reported case of SICH occurring during the progression of CSDH.
Chronic subdural hematoma (CSDH) is a common neurologic disease among the elderly. Spontaneous intracerebral hemorrhage (SICH) associated with CSDH has been reported as a rare complication.1,3,5,11,16) In most cases they develop during or after burr hole drainage, and are related to rapid decompression. Coagulopathy, vascular malformation, aneurysm, and neoplasm have been reported as other possible cautions of SICH development associated with CSDH.3,8,10,14) However, remote SICH that occurs during progression of CSDH has not been previously reported. Here we describe a case of SICH that occurred during the progression of CSDH, and discuss possible mechanisms, with clinical and radiologic findings.
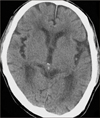
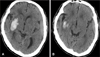
An 87-year-old man was admitted to the emergency de-martment due to vomiting, followed by quadriparesis. The patient was healthy before admission and his medical history was unremarkable. The patient hit his head after a motor vehicle accident one month prior to admission, and was admitted to another hospital. The initial brain computed tomography (CT) scan obtained at a local hospital revealed no abnormal findings other than brain atrophy (Figure 1). The patient was managed conservatively for 3 weeks, and discharged. He suffered from headache. He was observed at home, until a relapse of headache and quadriparesis, followed by neurological deterioration, led him to be transferred to our institution four weeks after trauma. On admission, the patient was stuporous with Glasgow coma scale (GCS) score 9 (E2V2M5), and motor examination revealed left hemiparesis (grade 1/5) and right hemiparesis (grade 3/5). Pupils were equal and reactive to light. Blood pressure in the emergency department was 210/130 mmHg. Brain CT scan showed an isodense SDH in the left hemisphere, with a maximum thickness of 1.5 centimeters and acute ICH in the right basal ganglia. Obliteration of the left ambient cistern and left brainstem compression due to the mass effect of CSDH was also revealed (Figure 2). Laboratory studies, including liver function tests, platelet count, prothrombin time and partial thromboplastin time, were all within normal limits.
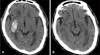
An emergency operation to drain the hematoma through a burr hole in the left frontal area was performed. The silicon tube was inserted into the hematoma immediately, and then connected to a closed drainage system. The dark-oil colored hematoma was drained intraoperatively. Intracranial pressure checked during the initial puncture was at approximately 23 centimeter H2O. The drainage tube was usually removed on the 3rd day after surgery. Surgical intervention was not attempted and conservative treatment was performed with the SICH. The patient was monitored closely with frequent neurologic examination, because rapid decompression might have increased the amount of ICH. Three days after surgery, a follow-up CT scan showed adequate evacuation of CSDH and no enlargement of ICH (Figure 3A). Blood pressure became gradually stable without antihypertensive medication. The neurologic examination revealed significant improvement of right hemiparesis and GCS score 14 (E4V4/M6). However, left hemiparesis was not improved. The left brainstem, which was compressed in the preoperative CT scan, was well visualized on the brain CT scan obtained on 14 days after surgery (Figure 3B). On three months after surgery, the patient was found to have slowly regained strength in his left arm and leg (grade 3/5) with physiotherapy.
SICH and CSDH is one of the most common types of intracranial hematoma. SICH associated with CSDH is a relatively uncommon neurosurgical complication, and is often associated with significant morbidity and mortality. SICH may be a development in various conditions. Chronic arterial hypertension is the most common cause, especially when this entity is located in putamen, thalamus, pons, and cerebellar vermis.6,7) Other possible reasons are brain tumor bleeding, coagulopathy, amyloid angiopathy, vascular malformation, drug abuse, and aneurysmal rupture. On the other hand, CSDH is associated with frequent head injury, chronic alcoholism, epilepsy, aging, use of anticoagulants, and prior brain atrophy. Therefore, the rarity of SICH which is associated with CSDH could be explained by etiologic differences.
Regarding pathogenesis, three possible types of SICH associated with CSDH could develop. First, the most common type of this condition is SICH occurring during or after evacuation of CSDH.1,3,5,11,16) The incidence of SICH occurrence after evacuation of CSDH has been reported as low as 1 to 5%.11) Regarding pathogenesis, diapedesis through increased permeability of parenchymal vessels due to the sudden increase in cerebral blood flow combined with defective cerebral autoregulation following the existence of a longstanding extracerebral mass is the most likely mechanism.4) Fragile cerebral vessels due to aging may be another factor for this situation.13) The drainage of hematoma produced a movement of the hemisphere that resulted in the rupture of a weak cerebral vessel.10) Chronic dilatation of small arterial vessels and loss of carbon dioxide reactivity in the ischemic hemisphere also contribute to pathogenesis.5,11) Slow decompression with a closed system for CSDH treatment can avoid this unpredictable complication.15)
Second, Hirakawa et al.8) reported on another type of CSDH with direct intracerebral rupture forming an acute subcortical hematoma. They speculated that fresh bleeding into the adjacent brain parenchyma directly resulted in subcortical ICH through a rupture of the inner membrane of CSDH. In this case, the patient had long-standing liver cirrhosis; therefore, the authors explained that disseminated intravascular coagulation played an important role in development of SICH. On the other hand, concurrent acute SDH was present in 20% of primary lobar ICH.12) However, no SDH were identified in deep ICH. Rupture of a leptomeningeal artery adjacent to the dura with extravasation into both brain parenchyma, as well as the subdural space, is the most likely explanation for the syndrome of SDH and nontraumatic primary lobar ICH. Most SDH were immediately adjacent to the lobar ICH and, all were in the same hemisphere.
Finally, remote or adjacent SICH from CSDH could also occur due to arterial hypertension, which might develop by CSDH progression with a mass effect on brainstem or increased intracranial pressure (IICP). Our case presented here was not consistent with previous observation of an association between SICH and SDH. Three possible mechanisms could be assumed; 1) SICH may have independently occurred during CSDH development and progression by each other mechanisms.; 2) If the brainstem was compressed by the mass effect of CSDH, blood pressure may have increased as a result of local ischemia on the brainstem, and SICH may have developed due to elevated blood pressure; 3) IICP caused by increasing CSDH decreased cerebral perfusion pressure, and reduction of cerebral perfusion pressure gradually resulted in an increase in blood pressure.2,9) So that eventually hypertensive SICH might have occurred. However, there is a slim chance of concurrence of CSDH and ICH randomly, making the other two mechanisms more likely. The second and third mechanisms are related to the Cushing response. The Cushing response is defined as arterial hypertension and bradycardia, which arises as a result of either generalized central nervous system (CNS) ischemia or local ischemia as a result of pressure on the brainstem. SICH could have developed independently from other etiologies, including atherosclerosis, tumor bleeding, or vascular malformation.
In our case, the initial brain CT scan showed no abnormal findings. However, a brain CT scan, obtained 1 month after trauma, revealed obliteration of the left ambient cistern, left hypothalamic herniation toward the upper midbrain, and left brainstem compression due to the mass effect of CSDH; nevertheless, acute SICH was located in the right basal ganglia. These radiologic findings could be evidence of left side brainstem compression by the mass effect of CSDH, which may have developed prior to development of SICH. Considering the second possible mechanism, SICH could have occurred as a result of arterial hypertension development. We reviewed admission records at the local hospital associated with vehicle accidents. In clinical findings, the patient's headache was much worse and cognitive impairment was newly developed in the hospital. Moreover, his blood pressure increased more than during the early hospital days following the motor vehicle accident. These clinical findings may be evidence of IICP caused by CSDH progression and acute changes in blood pressure and blood flow may lead to rupture of small penetrating arteries. The more sudden and severe change is the more likely the risk of rupture. Therefore, when we considered the third mechanism, we thought that hypertensive SICH might have developed as a result of IICP.
Rapid decompression of CSDH can increase the amount of intracerebral hemorrhage (ICH) due to rapid dynamic intracranial change. For avoiding this undesirable complication, we recommend slow decompression with burr-hole drainage for CSDH.
Figures and Tables
References
1. Akhaddar A, Ajja A, Elmostarchid B, Boucetta M. Combined epidural and intracerebral hematomas after evacuation of bilateral chronic subdural hematoma. Neurochirurgie. 2008; 54:728–730.

2. Avezaat CJ, van Eijndhoven JH, Wyper DJ. Cerebrospinal fluid pulse pressure and intracranial volume-pressure relationships. J Neurol Neurosurg Psychiatry. 1979; 42:687–700.

3. Chang SH, Yang SH, Son BC, Lee SW. Cerebellar hemorrhage after burr hole drainage of supratentorial chronic subdural hematoma. J Korean Neurosurg Soc. 2009; 46:592–595.

4. d'Avella D, De Blasi F, Rotilio A, Pensabene V, Pandolfo N. Intracerebral hematoma following evacuation of chronic subdural hematomas. Report of two cases. J Neurosurg. 1986; 65:710–712.
5. Dinc C, Iplikcioglu AC, Bikmaz K, Navruz Y. Intracerebral haemorrhage occurring at remote site following evacuation of chronic subdural haematoma. Acta Neurochir (Wien). 2008; 150:497–499. discussion 499.

6. Douglas MA, Haerer AF. Long-term prognosis of hypertensive intracerebral hemorrhage. Stroke. 1982; 13:488–491.

7. Harbaugh RE, Schlusselberg DS, Jeffery R, Hayden S, Cromwell LD, Pluta D. Three-dimensional computerized tomography angiography in the diagnosis of cerebrovascular disease. J Neurosurg. 1992; 76:408–414.

8. Hirakawa T, Tanaka A, Yoshinaga S, Ohkawa M, Tomonaga M. Calcified chronic subdural hematoma with intracerebral rupture forming a subcortical hematoma. A case report. Surg Neurol. 1989; 32:51–55.
9. Marmarou A, Shulman K, Rosende RM. A nonlinear analysis of the cerebrospinal fluid system and intracranial pressure dynamics. J Neurosurg. 1978; 48:332–344.

10. Miyazaki T, Matsumoto Y, Ohta F, Daisu M, Moritake K. A case of unknown origin subarachnoid hemorrhage immediately following drainage for chronic subdural hematoma. Kurume Med J. 2004; 51:163–167.

11. Modesti LM, Hodge CJ, Barnwell ML. Intracerebral hematoma after evacuation of chronic extracerebral fluid collections. Neurosurgery. 1982; 10:689–693.

12. Patel PV, FitzMaurice E, Nandigam RN, Auluck P, Viswanathan A, Goldstein JN, et al. Association of subdural hematoma with increased mortality in lobar intracerebral hemorrhage. Arch Neurol. 2009; 66:79–84.

13. Sousa J, Golash A, Vaz J, Chaudhary H. Spontaneous intracerebral haemorrhage following evacuation of chronic subdural hematomas. J Clin Neurosci. 2004; 11:794–796.

14. Stefini R, Ghitti F, Bergomi R, Catenacci E, Latronico N, Mortini P. Uncommon presentation of ruptured intracranial aneurysm during surgical evacuation of chronic subdural hematoma: case report. Surg Neurol. 2008; 69:89–92. discussion 92.





 PDF
PDF ePub
ePub Citation
Citation Print
Print


 XML Download
XML Download