Introduction
Seizure or epilepsy is infrequently reported in patients with chronic subdural hematoma (CSDH). Previous papers have been reported seizure rates ranging from 2% to 19% although the characteristics of the populations or descriptions of the seizure varied widely.2,6,10,14) The CSDH is known to have a favorable prognosis when managed with simple burr-hole trephination and drainage. Although the chance for recovery from CSDH is very likely, unexpected neurologic complication may occasionally occur in the postoperative course.
The authors report unusual case of focal seizure due to CSDH which was not controlled and progressed to status epilepticus in spite of open hematoma evacuation.
Case Report
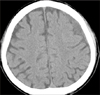
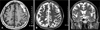
A 62-year-old man presented with focal seizure on the right perioral area and hand without loss of consciousness of 5-minute duration. His past history revealed a minimal head trauma about one month ago. Besides seizure, there was no neurologic deficit including mental change and motor weakness. Admission brain CT showed tiny amount of CSDH, with low density on the left fronto-parietal area, eliminating necessity of burrhole drainage (Figure 1). Thus, we provided him of anticonvulsant medication of phenytoin with loading dose of 1,000 mg via intravenous route. His perioral focal seizure, however, was not subsided, and valproic acid (900 mg) was added, but only to fail until 10th hospital days. Moreover, his seizure pattern was changed to complex partial seizure with occasional alteration of consciousness as a form of loss of consciousness or automatism. Subsequent, brain magnetic resonance image (MRI) revealed same finding as previous CT, but differed from previous CT in that motor cortex on the front of the central sulcus was compressed (Figure 2). Electroencephalogram (EEG) showed focal irregular theta and intermittent spike at the left central region. We thought that EEG findings were indicative of diffuse cerebral dysfunction related with structural lesion on the left hemisphere.
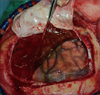
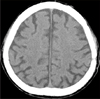
Craniotomy was performed for total evacuation of CSDH on 14th hospital day. Skin was incised using inverted U shape, and square craniotomy was performed above the CSDH. On the operative field, there was dark reddish hematoma capsule at the subdural space without adhesion to arachnoid membrane (Figure 3). Hematoma was clearly removed and meticulous bleeding control was performed. Immediate postoperative brain CT did not revealed any abnormal findings except brain swelling at the operative site (Figure 4). Pathologist confirmed hematoma combined with chronic inflammation.
After surgical treatment, focal seizure did not show up for 3 days despite sole use of valproic acid. However, in 4th postoperative day, focal seizure relapsed on the right perioral area and hand about 5 times per day. Thereafter, the frequency and duration of seizure was increased about 15-20 times per day and 30 minutes despite additional medication including full dosage of carbamazepine and clonazepam. Follow-up EEG revealed the presence of intermittent spikes and focal irregular theta at the left hemisphere. Finally, we concluded his diagnosis to status epilepticus and managed with coma therapy. Follow-up EEG of 5th day after coma therapy showed slightly improved pattern such as occasional spike or sharp waves at the left central area. And then, 10th day (after coma therapy) EEG revealed no epileptiform discharge. Two weeks after coma therapy, he did not suffer any more epileptic attack on the right hand and perioral area. The last brain CT revealed (Figure 5) complete absorption of hemorrhagic density. He has taken carbamazepine (200 mg) and valproic acid (600 mg) to prevent seizure re-attack.
Discussion
Following CSDH, the incidence of seizure occurrence has been reported variously by 2-19%, although CSDH happened from a minor head trauma above 80% of the patients.2,13) A main pathophysiologic mechanism of CSDH is continuous hematoma accumulation due to injury of vein without damage of dura mater, subarachnoid membrane and brain parenchyma. Therefore, it seems reasonable that seizure resulting from giving impulse to brain parenchyma is infrequent.7)
According to previous papers, the risk factors of seizure attack in CSDH patients are as followings; previous history of convulsion, heavy alcoholics, maintaining of post-traumatic stress syndrome, and repeated head trauma.3,5,8) Cole et al.5) reported that seizure occurrence rate was elevated up to 47% in heavy alcoholics with repeated head trauma. After head trauma, the risk of seizure is increased in proportion to degree of brain injury as followings; brain parenchyma injury, hemorrhage, and destruction of dura mater.3,8,12) If hemorrhagic contusion or intraparenchymal hemorrhage combined with CSDH, seizure occurrence was elevated up to 40%.4) However, the present case did not have any above mentioned risk factors on past history. Also, admission CT did not revealed any other hemorrhage but CSDH or destructive lesion of the dura and brain parenchyma.
Brodersen and Gjerris1) reported that hematoma drainage can decrease the seizure occurrence, because brain compression cause decreasing blood flow, rendering ischemia and leading to seizure. Kotwica and Brzeziński11) presented that irritated brain cortex by hematoma sac provoke convulsion, and for this reason, resection of the hematoma sac can reduce the seizure occurrence rate.
In our case, there was intermittent focal seizure from small amount of CSDH. His seizure was continued and aggravated despite small amount of CSDH without any other hemorrhagic density on initial brain CT scan and combined anticonvulsant therapy. Craniotomy was performed for hematoma evacuation after identification of seizure focus from EEG and brain compression from MRI. Although appropriate anticonvulsant and surgical hematoma evacuation, seizure was not controlled and still progressed to status epilepticus. Seizure aggravation and generalization may be due to a paradoxical reaction or drug-induced encephalopathy, sedative effects or inappropriate use of a drug.9) Experimental and clinical data, also, indicate that epilepsy and seizures leading to neuronal cell loss and irreversible brain damage, neuro-degeneration, can be a seizure focus.15) In authors' opinion, his aggravated symptom was caused by paradoxical drug reaction and neuro-degeneration, so early stage surgical intervention may helpful to prevent from seizure progressing to status epilepticus.
In the present case, there may be two problems during management of seizure control. First, there was a mistake associated with selection of antiepileptic drug. Phenytoin as first medication may be wrong choice or not enough concentrated for seizure control in the blood. Second, there may be a wrong selection of the operative method. We chose the craniotomy for hematoma removal, not burr-hole trephination due to some reasons as follows; There was a possibility of brain damage during catheterization following burr-hole trephination and mal-position of burr-hole site due to small amount. However, it is definite that brain manipulation is more frequent for meticulous bleeding control during the craniotomy procedure than burr-hole trephination. In other words, the cause of progression to status epilepticus may be more during or after hematoma removal. So, there would be more preferable outcome with burr-hole trephination than craniotomy in spite of risks as above mentioned, using stereotactic apparatus.
Conclusion
We experienced uncontrolled seizure which developed from small amount CSDH despite hematoma evacuation and progressed to status epilepticus. We should be alert that there is a necessity of early surgical intervention when there is a definite compressive lesion on the brain cortex and EEG findings, despite small amount of CSDH, in patients with presentation of seizure.




 PDF
PDF ePub
ePub Citation
Citation Print
Print



 XML Download
XML Download