Abstract
Objective
In pediatric patients, autologous-bone assisted cranioplasty is preferred because the child's original skull material will become reintegrated. Unfortunately, the replaced bone flap sometimes undergoes bone resorption, which results in structural breakdown necessitating reoperation. We report two children who underwent failure of autologous cranioplsty following decompressive craniectomy.
Methods
An 11-year-old girl visited our emergency department with the chief complaint of stuporous mental change. Radiologic evaluations identified intracranial hemorrhage with arteriovenous malformation (AVM) in left fronto-parietal lobe. Decopmressive craniectomy and clipping of nidus of AVM was performed on the day on admission. After 1 month later, the autologous-bone assisted cranioplasty was performed. An 11-year-old boy visited our emergency department after trauma. The computed tomography (CT) scan revealed acute subdural hematoma in left cerebral convexity. Decompressive craniectomy was performed immediately. After 3 months later, the autologous-bone assisted cranioplasty was performed.
Results
One year, and 2 months respectively after cranioplasty, they revisited outpatient service center with the chief complaint of breakdown of skull contour. The three dimensional CT scan revealed resorption of autologous bone graft. Repair was performed using porous polyethylene implant (Medpor®, Porex Surgical, Inc, Newnan, GA, USA) and absorbable microplates.
In many hospitals, decompressive craniectomy is undertaken for treating severe cerebral swelling due to spontaneous intracranial hemorrhage (ICH) or trauma. Delayed craniplasty is then performed to reconstruct skull contour after the brain edema has resolved.
Numerous techniques and alloplastic materials have been developed to alternate autologous bone and to reduce complications after cranioplasty. However, in pediatric patients, placement of original bone flap which is removed in craniectomy is preferred because the potentiality of reintegration with resident bone as he or she grows up.
Unfortunately, the replaced bone flap sometimes undergoes bone resorption, which results in structural breakdown necessitating reoperation. We present two cases which of failure of autologous bone assisted caranioplasty following decompressive craniectomy in children.
An 11-year-old girl was referred to our emergency department with stuporous mentality. She had a Glasgow Coma Score (GCS) of 7 (eye opening 1, verbal response 1 and motor response 5) points with right side motor weakness.
A head computed tomography (CT) scan confirmed the ICH in left fronto-parietal lobe with severe brain parenchymal edema. An enhanced CT was subsequently performed, which demonstrated an arteriovenous malformation (AVM) nidus in ICH region.
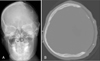
Shortly after the CT study, decompressive craniectomy, direct evacuation of hematoma and removal of AVM nidus was performed. The size of skull defect area was about 98.5 cm2 and the thickness of bone flap was 3.0 mm (Figure 1).
The bone flap was irrigated and debrided with normal saline, dried, wrapped in a sterile gauze and glove, and covered with sterile cotton. Then the bone temperature was reduced quickly and suddenly to -70 to -80℃ and then stored in laboratory refrigerator.
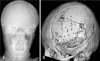
One month after craniectomy, cerebral swelling was resolved and autologous-bone assisted cranioplasty was performed (Figure 2). Before one day of the operation, the bone flap was transferred to operation room, irrigated with normal saline and sterilized with Ethylen Oxide in autoclave for 12 hours. The bone was fixed using absorbable microplate and microscrews, and the scalp was closed layer by layer using absorbable vicryl galeal sutures and nylon skin sutures. Post-operative antibiotic therapy was continued for 7 days.
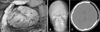
After 1 year after carnioplasty, she revisited outpatient service center with the chief complaint of breakdown of skull contour. The three dimensional CT scan revealed resorption of autogenous bone graft (Figure 3). Repair was performed using porous polyethylene implant (Medpor®, Porex Surgical, Inc, Newnan, GA, USA), absorbable microplates and microscrews (Figure 4). At the time of cranioplasty revision, there was no infection sign, and the loose bone pieces were removed totally and irrigated massively.
She has been followed up with no complication during 6 months after second craniopalsty.
An 11-year-old boy was referred to our emergency department with stuporous mentality after violence. A CT scan revealed the acute subdural hematoma (SDH) in left cerebral convexity with midline shifting.
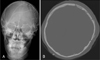
Immediately after the CT scan, decompressive craniectomy and removal of hematoma was performed. The size of skull defect area was about 105.2 cm2 and the thickness of bone flap was about 3.5 mm (Figure 5). The bone flap was preserved in the same way, as mentioned above, until the child would be ready for cranioplasty.
Three months after craniectomy, the autologous-bone assisted cranioplasty was performed (Figure 6). The operation technique and post-operative care was same with previous case.
After 2 months after carnioplasty, he revisited outpatient service center with the chief complaint of breakdown of skull contour. The three dimensional CT scan revealed resorption of autogenous bone graft (Figure 7). In the operation room, the fragile and dried bone pieces were removed, and then porous polyethylene implant (Medpor®) was fixed by absorbable microplates and microscrews (Figure 8).
The pathologic finding was broad necrosis with dystrophic calcification and chronic inflammation with fibrosis and foreign body reaction (Figure 9).
He has been followed up with no complication during 5 months after second craniopalsty.
In pediatric patients, delayed cranioplasty presents a dilemma: should the surgeon use autologous bone or one of various other materials?3,7) The merits of using autologous bone include an easy return of the former cranial contour, and no need to introduce foreign materials.14) Autologous bone cranioplasty has the obvious advantages of lack of immune reaction and absence risk of disease transmission. Furthermore, it has a potentiality to reintergrate with resident bone and to grow up.5)
On the other hand, historically, the skull is one of the most difficult regions in which to use autograft because of the propensity for resorption.7,12) Published data concerning the results of cranioplasty in children are limited, but some analyses of those reported high resorption rate after autologous cranioplasty in children compared to adults.5) Especially, in cases of autoclaved bone, resorption rate can be as high as 25-40 %.1,11)
Survival of a bone implantation graft depends on the reaction of the surrounding tissue and on functional contact between cancellous bone and adjacent resident bone.14)
Bone graft healing is complex and involves processes of revascularization, osteoconduction, osteoinduction, and osteogenesis. During the first week after grafting, capillaries from surrounding bone diploe, dura and scalp infiltrate the transplant bed (revascularization). During the second week, fibrous granulation tissue proliferates and osteoplastic activity occurs. Primitive mesenchymal cells differentiate into osteoprogenitor cells, a process nowadays termed osteoinduction, and subsequently these osteoprogenitor cells differentiate into osteoblasts that are capable of forming new bone to replace the necrotic bone which is gradually absorbed.1) Osteoconduction is the process whereby osteoprogenitor cells from the surrounding tissue migrate into the three-dimensional structure of bony and protein matrix. It is now understood that auto- and allo-grafts have relied on osteconduction as the main principle of cranioplasty. It is also understood that, by contrast, in osteoinduction, cells do not have to migrate from the surrounding tissues but, probably with the help of bone morphogenetic proteins, can be produced in situ.15) Osteogenesis involves new bone formation by surviving preosteoblasts within the graft. As the healing progresses, the bone graft is remodeled through bone resorption and new bone formation.14)
The duration (between failure and replacement) and method of bone flap preservation have previously been suggested to lead to breakdown of the bone flap.13) Autoclaving of the bone flap has been shown to denature bone protein and impair vascularization and resorption, and therefore, is not routinely performed.8) In a recent study, investigators reevaluated the use of fresh-frozen autologous bone flaps in patients undergoing delayed cranioplasty; they reported bone resorption in only one (4%) of 49 cases 15 months following cranioplasty.9)
Other authors have suggested that patients retain an increased propensity for skull healing when cranioplasty is performed prior to puberty.6) Grant et al.5) reported that symptomatic bone resorption subsequently occurred in 20 children (50%) in all of 40 children. The incidence of bone resorption significantly correlated with an increased skull defect area. No significant correlation was found with age, sex, or anatomical location of the skull de-fect, number of fractured bone fragments, presence of a shunt, cause for decompressive craniectomy, method of duraplasty, or interval between the craniectomy and the cranioplasty.5)
In our 2 cases, the duration between failure and first replacement was not long. Patients' age might not be risk factor of resorption because they were prior to puberty. Autoclaving of bone might be included to risk factor, but not reasonable. Because, in 100 cases of adult cranioplasty, there were no cases of resorption in the same technique of bone preservation in our hospital.
We hypothesize that these 2 cases of resorptions in children were due to the thinness (3.0 and 3.5 mm) of the calvaria and large defect area (98.5 and 105.2 cm2).
Loder10) reported that the left postero-lateral skull thickness of children was 2.4-4.8 mm (average 3.6). The skull thickness of our children was absolutely thinner than that of adults, and relatively thinner than that of other childlen.
Gerant et al.5) reported that defects greater than 75 cm2 had a failure rate greater than 60% whereas those smaller than 75 cm2 were associated with no failures.
Although autologous bone graft has been the preferred material, the associated rate of failure and high rates of reoperation in patients with large defects and thinness suggest that alternative solutions might be considered at the time of initial cranioplasty.
On the other hand, several techniques are known to be available for preventing autologus bone resorption. At the time of cranioplasty, drilling down the edges of both the donor and recipient bones and overlap the edges as much as possible using a tongue-and-groove technique to maximize bone-bone contact and promote osteoblastic ingrowth.5) Some studies involving nonvascularized autologous bone grafts have shown that rigid fixation of the graft is critical to minimize graft resorption and facilitate osteoconduction.4) The normal microenvironment of the cranium is not sufficient to promote osteogenesis and may require the addition of growth factors to recruit cells and stimulate bone repair.5) Researchers have studied the use of bone growth factors including insulinlikegrowth factor-I, transforming growth factor-β1, and other growth factors can be helpful to osteogenesis.2)
Figures and Tables
FIGURE 1
Images of pre- and post-craniectomy. A: Enhanced brain computed tomography (CT) revealed intracranial hemorrhage with AVM nidus in left frontoparietal lobe. B-D: Skull X-rays and CT showed left craniectomy state. AVM: arteriovenous malformation.
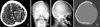
FIGURE 5
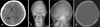
Images of pre- and post-craniectomy. A: CT revealed acute subdural hematoma and severe brain parenchymal edema in left cerebral convexity. B-D: Skull X-rays and CT showed left craniectomy state.
References
1. Apuzzo MLJ. Brain Surgery. 1st ed. New York: Churchill Livingstone;1993. p. 1385–1393.
2. Arnaud E. Advances in cranioplasty with osteoinductive biomaterials: summary of experimental studies and clinical prospects. Childs Nerv Syst. 2000; 16:659–668.

4. Citardi MJ, Friedman CD. Nonvascularized autogenous bone grafts for craniofacial skeletal augmentation and replacement. Otolaryngol Clin North Am. 1994; 27:891–910.

5. Grant GA, Jolley M, Ellenbogen RG, Roberts TS, Gruss JR, Loeser JD. Failure of autologous bone-assisted cranioplasty following decompressive craniectomy in children and adolescents. J Neurosurg. 2004; 100:2 Suppl Pediatrics. 163–168.

6. Gruber R, Peter R, Hora J. The prognosis of cranioplasty following large craniectomy in children. Z Kinderchir. 1988; 43:375–383.

7. Hockley AD, Goldin JH, Wake MJ, Iqbal J. Skull repair in children. Pediatr Neurosurg. 1990-1991; 16:271–275.

8. Itoh Y. Clinicopathological study of cranioplasty using freeze preserved autogenous skull[in Japanese]. J Tokyo Med Coll. 1991; 49:550–564.
9. Iwama T, Yamada J, Imai S, Shinoda J, Funakoshi T, Sakai N. The use of frozen autogenous bone flaps in delayed cranioplasty revisited. Neurosurgery. 2003; 52:591–596.

10. Loder RT. Skull thickness and halo-pin placement in children: the effects of race, gender, and laterality. J Pediatr Orthop. 1996; 16:340–343.

11. Manson PN, Crawley WA, Hoopes JE. Frontal cranioplasty: risk factors and choice of cranial vault reconstructive material. Plast Reconstr Surg. 1986; 77:888–904.
12. Pochon JP, Klöti J. Cranioplasty for acquired skull defects in children--a comparison between autologous material and methylmethacrylate 1974-1990. Eur J Pediatr Surg. 1991; 1:199–201.
13. Prolo DJ, Oklund SA. The use of bone grafts and alloplastic materials in cranioplasty. Clin Orthop Relat Res. 1991; (268):270–278.
14. Robert M. Redfern, Heinke Pul: Cranioplasty. Adv Clin Neurosci Rehabil. 2007; 7:32–34.




 PDF
PDF ePub
ePub Citation
Citation Print
Print


 XML Download
XML Download