Abstract
Objective
Carotid cavernous fistulae (CCF) after head traumas are abnormal communications between the internal carotid artery and venous compartments of the cavernous sinus. Fistulae are uncommon but well-documented sequelae of craniofacial trauma. We report our experiences in placing detachable coils in the management of rare cases of direct CCF after head injuries.
Methods
From January 2002 to December 2007, nine patients were admitted and treated for traumatic direct CCF at our hospital. The medical records and neuroimaging studies were retrospectively reviewed.
Results
All 9 patients had previous head trauma histories and ocular symptoms including exophthalmos, conjunctiva injection, and chemosis. Angiography was performed to confirm the CCF and for treatment planning. According to the angiograph, three patients had pseudo-aneurysms. All of the patients were treated with detachable coils via the trans-arterial endovascular maneuver. Among them, two patients had recurrences, the first at postoperative day one and the other eleven days after the surgery. Both recurrences were retreated endovascularly. All nine patients were successfully treated anigographically by means of trans-arterial embolization and improved symptomatically within a week.
Conclusion
The etiology of CCF, especially type A, is well known. Also, the advances in interventional neuroradiology and cranial base surgery are chiefly responsible for the evolution of treatment strategies for CCF. Although other techniques and approaches with other materials may be useful, embolization with detachable coiling, as used in our cases, can be a safe and effective method to occlude the fistula and to improve symptoms.
Carotid cavernous fistulae (CCF) are abnormal communications between the internal carotid artery (ICA) and venous compartments of the cavernous sinus. According to Barrow's classification, most of type A CCF are high flow and traumatic in origin.3) The etiology of a CCF can be traumatic and due to a skull base injury with a direct tear of the cavernous part of the ICA, or spontaneous and due to an intracavernous rupture of an aneurysm arising from a persistent primitive trigeminal artery. CCF may also be associated with cavernous sinus pathology such as arteriosclerotic changes of the arterial wall, fibromuscular dysplasia or Ehler-Danlos syndrome.8,9) Type A CCF develop as a complication of cranial base trauma in 0.2-0.3% of craniofacial trauma patients.9) The treatment for CCF is conducted via open craniotomy or endovascular therapy. The choice of treatment is often made by the operator on a case-by-case basis. Endovascular therapy involves embolizing the patency of the abnormal communication using a detachable balloon or coil material with or without a stent-as-sisted technique.
Although a traumatic CCF is a rare complication of traumatic brain and facial injuries, it can result in significant morbidities such as motor paralysis, unconsciousness, and blindness as the disease progresses or mortality.5,12) We report our experiences in placing detachable coils in the management of these rare cases of direct CCF after head traumas.
Between January 2002 and December 2007, 1,563 patients were admitted to our department due to head trauma, and among them, traumatic CCF type A occurred in 9 patients (0.57%). All 9 patients were treated with endovascular detachable coils, via the trans-arterial approach. A retrospective review of all of the images and the records of these patients was performed (Table 1).
All of the patients had a history of head trauma and ocular symptoms including exophthalmos, bruits, and chemosis. Symptoms that were not present at the time of the head trauma were revealed between 1 and 60 months later (mean duration; 12.8 months). There were uncommon symptoms, including cranial nerve deficits (n=1), brain stem signs such as a gait disturbance (n=1), mental deterioration (n=1), diplopia (n=1), blindness (n=2), limited eyeball movement (n=1), vanishing of the papillary light reflex (n=2), and severe epistaxis (n=1). Mental deterioration was the main symptom in one patient who had an intracerebral hemorrhage in the left frontotemporal area, because of bleeding from a pseudoaneurysm.
Computed tomoangiography (CTA) and conventional angiography studies were done to confirm the CCF. Initially, the CCF was detected by CTA, and its type was confirmed by conventional angiography. All of the cases had abnormal connections from the cavernous part of the ICA to the cavernous sinus. The fistula was drained via variable veins including the inferior petrosal sinus (9/9), the superior petrosal sinus (7/9), and the superior ophthalmic vein (9/9). A pseudoaneurysm was noted in 3 patients; two of them had a fistula connecting the cavernous portion of the ICA to the cavernous sinus, and one from the base of the ophthalmic artery to the cavernous sinus.
All of the patients received endovascular treatment using a detachable coil. The right femoral artery was used for the trans-arterial approach and punctured using the Seldinger technique in all of the patients. The carotid cavernous fistula was occluded after the detachable coil embolization of the defect in the vessel wall. Two of the three patients who had pseudoaneurysms in the cavernous portion of the ICA had to have a total occlusion of the ICA after testing the tolerability with the balloon occlusion test (BOT). They showed no neurologic deficit during the BOT and no electroencephalogram (EEG) changes. Contralateral ICA angiography revealed the venous filling for both hemispheres. One other patient who had a pseudoaneurysm in the ophthalmic artery base underwent endovascular coiling without an ICA occlusion.
Eight patients had their fistula completely obliterated at the end of the first procedure with the detachable coil embolization using the trans-arterial approach (Table 2). The one remaining patient needed a second coiling the next day because the first procedure showed a partial occlusion, but a remnant CCF was also obliterated completely at the end of the second procedure. One patient's procedure was unsuccessful after the first endovascular coiling, and the CCF remained because it was in the pre-embolization state. Another patient's ocular symptoms and limited eyeball movement did not improve. This patient was retreated on the following day with an endovascular treatment via the transarterial approach. After the second procedure, his symptoms were completely relieved by the second postoperative day. One of the eight patients, who were successfully treated after the first endovascular coiling, had their symptoms completely relieved after the treatment, but 11 days later, a bruit reemerged, and a repeat angiography showed a recurrence of the CCF. A recoiling procedure was carried out and the bruit was gone on the day after the recoiling. Seven patients' ICA could be preserved, but on the other hand, 2 patients' ICA were complete occluded. The two that were occluded had pseudo-aneurysms. Another patient with a pseudoaneurysm on the ophthalmic artery was successfully treated by coiling without sacrificing the ICA. All of the patients completely recovered from their ocular and other symptoms within the first week after the treatment. All patients were followed for 6 to 24 months using MRI or angiography and showed no evidence of recurrence.
Four patients with direct type A CCF had a history of head trauma at 5, 28, 4, and 12 months prior to their visit to the Outpatient Department of Neurosurgery because of ophthalmic symptoms after the head trauma. And 4 patients were treated with endovascular coil embolization through the trans-arterial approach (Figure 1). After the treatment with the endovascular coiling, those patients' symptoms were relieved immediately. All of the patients were followed up between 6 and 12 months later and were evaluated for any clinical signs or symptoms. No recurrence of the ocular symptoms was noted during the follow-up period.
Two patients' ophthalmic symptoms developed 2 and 1 months after the head trauma, respectively. In case 5, after the end of first coil embolization, there was only a partial occlusion of the fistula, and the patient's symptoms were still present. On the next day, a second procedure was done via the same approach, and after the re-coiling, his symptoms improved on post operative day one. The patient in case 6 had a complete occlusion of the CCF defect after the first endovascular coiling and showed improvement of his ophthalmic symptoms. However, the bruit reemerged on the 11th day after the coil embolization (Figure 2). Recurrence of the CCF was confirmed by angiography, and recoiling via the arterial approach was repeated. The bruit disappeared the day after the recoiling. These two patients' ophthalmic symptoms were relieved after retreatment of the CCF with the detachable coil, and no more ophthalmic symptoms were detected during the following period of 2 years symptomatically and 1 year anigographically.
Three patients had a CCF type A with an associated pseudoaneurysm. The CCF and pseudoaneurysm of the patient in case 7 was treated without ICA occlusion with the detachable coil. The pseudoaneurysm on the ophthalmic portion was completely packed by the coil (Figure 3). Although the patient was symptom-free, angiography was followed up after 6 months and showed no remnant or recurrent fistula, but the pseudoaneurysm on the right ophthalmic artery was noted with coil compaction. This patient was treated using the endovascular detachable coil again on the coil-compacted pseudoaneurysm. In cases 8 and 9, the CCF and pseudoaneurysm were treated by sacrificing the ICA with an occlusion using the detachable coil. Before the ICA occlusion of the ipsilateral ICA, a BOT was performed to check the tolerability of the ICA occlusion. This test was done under EEG monitoring and continuous neurologic examination by means of contralateral ICA angiography. These patients did not show any EEG changes or any neurologic deficits. Additionally, the venous filling of the hemisphere with the lesion occurred in fewer than 2 seconds during the contralateral ICA angiography. And patients were taken the post-embolization perfusion computer tomography (CT). That was revealed no perfusion defect in both hemisphere (Figure 3D, E, F).
One patient, case 8, was followed up at 6 months after the coiling with angiography and for 2 years clinically. The angiography showed no CCF and the completely occluded ICA. The patient complained of no clinical symptoms associated with the ICA occlusion. The other patient, case 9, was lost during our follow-up period.
Barrow et al.3) classified CCFs into four types: Type A fistulas are direct, high-flow shunts between the ICA and the cavernous sinus, which usually occur after trauma or rupture of a caroticocavernous aneurysm, which usually occurs spontaneously; Type B fistulas are dural shunts between the meningeal branches of the ICA and the cavernous sinus; Type C are dural shunts between the meningeal branches of the external carotid artery (ECA) and the cavernous sinus; and Type D are dural shunts between the meningeal branches of both the ICA and ECA and the cavernous sinus.
Type B, C, and D lesions are low-flow dural fistulas that are spontaneous in origin, often idiopathic, and have a tendency to resolve spontaneously.14) Although type A CCF could develop spontaneously (e.g. pseudoaneurysm), type A CCF develop mainly after traumas. The cause of CCF development after trauma is thought mainly to be penetrating craniocervical injuries where direct trauma results when the vessel is in the path of a penetrating object or in proximity to an adjacent fracture. The treatment methods for type A CCF can be divided into microsurgery, endovascular surgery, and radiosurgery.
Surgical treatment of the CCF has been attempted since the beginning of the 17th century. In 1973, Parkinson reported a successful direct surgical repair of a direct CCF with preservation of the parent artery.25) However, the technical difficulty and high invasiveness of this procedure have precluded the wide adoption of this surgical treatment.
Endovascular therapy is considered the first line and most important treatment tool in all types of CCF patients.6,31) It is divided into the trans-arterial or trans-venous method according to the vessel approached and into a balloon or coil embolization with or without stent assistance according to the material used.3,11,13,16,19,24,31) Serbinenko reported on the use of the detachable balloon method in CCF patients. By this method, the CCF is occluded with the detachable balloon method through the transarterial or transvenous approach.7,10,20,21) This treatment showed less morbidity and mortality than surgical treatments, so it was used as the first endovascular treatment material.23) However, because the patients' ocular palsy did not improve and the CCF often recurred due to deflation and displacement of the balloon, this method is seldom used currently. Recently, the detachable coil has gained wide acceptance because it has some advantages, such as being useful for very small CCF lesions and allowing for the deployment of the coil inside the cavernous sinus. In our institution, this method was used in the patients with ICA aneurysms.
However, this detachable coil method has some difficulties when attempting to match it to the wall morphology or flow of the fistula in some selective cases. Therefore, the stent assistant method was co-used for supplementation in the selective cases where the CCF could not be occluded with only the coil because of the wall morphology or flow of the fistula. This method may provide more stability because the insertion of the stent into the parent vessel is accomplished when the coil or balloon is applied to the CCF defect site. This method was used mainly in wideneck true and false aneurysm cases.17,26-28) The choice of endovascular stent placement combined with the detachable coil has been advocated.2,4,18) Radiosurgery has a role in the treatment of type B, C, and D CCF, but is not indicated in the type A CCF because of the high flow and large size of the fistula.
In our 8th and 9th cases, the CCF was present with a pseudoaneurysm, and the ICA and CCF were totally occluded. Before the total occlusion of a lesion on the ICA, we assessed the patients with a BOT to confirm their ability to tolerate the occlusion. Endovascular techniques for the BOT of parent vessels have been developed to assess the vascular reserve of a particular vascular territory with neurologic monitoring before an arterial sacrifice.30) During the BOT, direct or indirect cerebral blood flow was estimated by several tests, such as EEG monitoring, Xenon CT, trans cranial doppler (TCD), and neurologic examinations. Linskey et al.15) promoted the use of stable Xenon CT to predict outcomes after carotid artery occlusion. They identified the low-risk patients, who were defined as having a blood flow greater than 30 mL/100 g/minute during a BOT. Abud et al.1) argued the tolerability through angiographic evaluation during a BOT, which was a simple method for evaluating tolerability. They suggested that permanent ICA occlusion will be safe when venous drainage delay in the BOT is not more than 2 seconds. Furthermore, the TCD technique was used as a noninvasive method. Sacrificing the ICA is feasible when the occlusion test results in an ipsilateral initial reduction in the velocity of the middle cerebral artery to ≥60% of pre occlusion values.29) Also, EEG was used to estimate the tolerability for the occlusion. During the BOT, EEG wave changes suggest that an ICA occlusion could induce cerebral infarction.22) Other methods of checking the BOT can be done by using the pharmacological hypotensive challenge test and 99mTc hexamethylpropylene amine oxamine (HMPAO). In our case 8, the ICA could be sacrificed because the patient tolerated the occlusion as evidence by the findings from EEG monitoring. Also, this patient did not show any neurologic deficits during the follow-up period. In case 9, the patient's tolerability was assessed by using a contra-lateral ICA angiography test and neurologic examinations. The venous drainage of both hemispheres was not delayed more than 2 seconds in the BOT, and no new neurologic deficits were noted. After confirming the tolerability, the ipsilateral ICA was sacrificed, and there was no evidence of a cerebral infarction in the postoperative perfusion brain CT.
Currently, the choice of a treatment modality for type A CCF is controversial, but one could make a decision according to the location, the presence of a coexisting pseudoaneurysm, and the BOT results.
Although a traumatic CCF is a rare complication of traumatic brain and facial injuries, it can result in significant morbidities or mortality. With the advances in interventional neuroradiology, remobilization with detachable coiling as in our cases can be a safe and effective method to occlude type A CCF and to improve CCF related ocular symptoms.
Figures and Tables
FIGURE 1
Pre- and post-operative arterial phase of the ICA angiogram in the case 2 patient. A and B: AP and lateral view of the pre-operative angiogram. Left ophthalmic vein, middle cerebral vein, inferior petrosal sinus, and pterygoid plexus are prominent in the arterial phase suggesting a high-flow CCF. C and D: AP and lateral view of the post-operative angiogram. Complete occlusion state of the CCF. CCF: carotid cavernous fistulae. ICA: internal carotid artery.
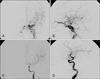
FIGURE 2
Pre- and post-operative arterial phase of the ICA angiogram in the case 6 patient. A: Pre-operative angiogram shows a prominent left ophthalmic vein, middle cerebral vein, inferior petrosal sinus, and pterygoid plexus. B: Post-operative angiogram shows complete occlusion of the CCF. C: Recurrence of the CCF is noted after the first embolization. D: Complete occlusion of the CCF after the 2nd procedure. CCF: carotid cavernous fistulae.
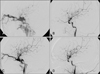
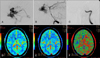
FIGURE 3
Pre- and post-operative arterial phase of the ICA angiogram and brain CT perfusion in the case 9 patient. A: Pseudoaneurysm and remarkable venous dilatation of the cavernous sinus including the petrosal sinus and pterygoid plexus is noted. B: Vertebral angiogram with manual compression of both the ICAs showing an aneurysm and fistulous drainage into the cavernous sinus. C: Complete occlusion of the ICA at the level of the aneurysm and complete obliteration of the aneurysm and CCF after the post-operative angiogram. D and F: Postembolization brain perfusion CT was revealed no perfusion defect in both hemisphere (D: Cerebral blood volume image, E: Cerebral blood flow image, F: Mean transit time image). ICA: internal carotid artery.
References
1. Abud DG, Spelle L, Piotin M, Mounayer C, Vanzin J, Moret J. Venous phase timing dura in balloon test occlusion as a criterion for permanent internal carotid artery sacrifice. AJNR Am J Neuroradiol. 2005; 26:2602–2609.
2. Auyeung KM, Lui W, Chow L, Chan F. Massive epistaxis related to petrous carotid artery pseudoaneurysm after radiation therapy: emergency treatment with covered stent in two cases. AJNR Am J Neuroradiol. 2003; 24:1449–1452.
3. Barrow DL, Spector R, Braun I, Tindall S, Tindall G. Classification and treatment of spontaneous carotid cavernous sinus fistulas. J Neurosurg. 1985; 62:248–256.
4. Cheng KM, Chan C, Cheung Y, Chiu H, Tang K, Law C. Endovascular treatment of radiation-induced petrous internal carotid artery aneurysm presenting with acute haemorrhage: a report of two cases. Acta Neurochir (Wien). 2001; 143:351–355.

5. d'Angelo VA, Monte V, Scialfa G, Fiumara E, Scotti G. Intracerebral venous hemorrhage in "high-risk" carotid-cavernous fistula. Surg Neurol. 1988; 30:387–390.
6. Day JD, Fukushima T. Direct microsurgery of dural arteriovenous malformation type carotid-cavernous sinus fistula: Indications, technique, and results. Neurosurgery. 1997; 41:1119–1124.
7. Debrun GM. Treatment of traumatic carotid-cavernous fistula using detachable balloon catheters. AJNR Am J Neuroradiol. 1983; 4:355–356.
8. Debrun GM, Vinuela F, Fox A, Davis K, Ahn H. Indications for treatment and classification of 132 carotid cavernous fistulas. Neurosurgery. 1988; 22:285–289.
9. Fabian TS, Woody J, Ciraulo D, Lett E, Phlegar R, Barker D, et al. Posttraumatic carotid cavernous fistula: frequency analysis of signs, symptoms, and disability outcomes after angiographic embolization. J Trauma. 1999; 47:275–281.
10. Forsting M, Sartor K. Hema and latex: a dangerous combination? Neuroradiology. 1991; 33:338–340.

11. Hara T, Hamada J, Kay Y, Ushio Y. Surgical transvenous embolization of a carotid-cavernous fistula with cortical drainage via a petrosal vein: two technical case reports. Neurosurgery. 2002; 50:1380–1383.
12. Iida K, Uozumi T, Arita K, Nakahara T, Ohba S, Satoh H. Steal phenomenon in a traumatic carotid-cavernous fistula. J Trauma. 1995; 39:1015–1017.

13. Lewis AI, Tomsick T, Tew JJ. Management of 100 consecutive direct carotid-cavernous fistulas: results of treatment with detachable balloons. Neurosurgery. 1995; 36:239–244.
14. Lewis AI, Tomsick T, Tew JM. Management of 100 consecutive direct carotid-cavernous fistulas: results of treatment with dieachable balloons. Neurosurgery. 1995; 36:239–245.
15. Linskey ME, Jungreis C, Yonas H, Hirsch WJ, Sekhar L, Horton J, et al. Stroke risk after abrupt internal carotid artery sacrifice: accuracy of preoperative assessment with balloon test occlusion and stable xenon-enhanced CT. Am J Neuroradiol. 1994; 15:829–843.
16. Manelfe C, Berenstein A. Treatment of carotid cavernous fistula by venous approach: report of two cases. J Neuroradiol. 1980; 7:13–19.
17. Men S, Ozturk H, Hekimoglu B, Sekerci Z. Traumatic carotid cavernous fistula treated by combined transarterial and transvenous coil embolization and associated cavernous internal carotid artery dissection treated with stent placement. J Neurosurg. 2003; 99:584–586.
18. Mericle RA, Lanzino G, Wakhioo A, Guterman L, Hopkins L. Stenting and secondary coiling of intracranial internal carotid artery aneurysm: technical case report. Neurosurgery. 1998; 43:1229–1234.

19. Miller NR, Monsieu L, Debrum G, Tamargo R, Nauta H. Treatment of carotid cavernous sinus fistula using a superior ophthalmic vein approach. J Neurosurg. 1995; 83:838–842.
20. Miyachi S, Negoro M, Handa T, Terashima K, Keino H, Sugita K. Histopathological study of balloon embolization: silicone versus latex. Neurosurgery. 1992; 30:483–489.
21. Monsein LH, Debrun G, Chazaly J. Hydroxyethyl methylacrylate and latex balloons. Am J Neuroradiol. 1990; 11:663–664.
22. Morioka T, Matsushima T, Fujii K, Kukui M, Hasuo K, Hisashi K. Balloon test occlusion of the internal carotid artery with monitoring of compressed spectral arrays (CSAs) of electroencephalogram. Acta Neurochirurgica. 1989; 101:29–34.

23. Morris PP. Balloon reconstructive technique for the treatment of a carotid cavernous fistula. AJNR Am J Neuroradiol. 1999; 20:1107–1109.
24. Mullan S. Treatment of carotid cavernous fistula by cavernous sinus occlusion. J Neurosurg. 1979; 50:131–144.
25. Parkinson D. Carotid cavernous fistula: direct repair with preservation of the carotid artery-technical note. J Neurosurg. 1973; 38:99–106.
26. Perez-Cruet MJ, Patwardhan R, Mawad M, Rose J. Treatment of dissecting pseudoaneurysm of the cervical internal carotid artery using a wall stent and detachable coils: case report. Neurosurgery. 1997; 40:622–626.

27. Phatouros CC, Sasaki T, Higashida R, Malek A, Meyers P, Dowd C, et al. Stent-supported coil embolization: the treatment of fusiform and wide-neck aneurysms and pseudoaneurysms. Neurosurgery. 2000; 47:107–115.

28. Redekop G, Marotta T, Weill A. Treatment of traumatic aneurysms and arteriovenous fistulas of the skull base by using endovascular stents. J Neurosurg. 2001; 95:412–419.

29. Sorteberg A, Sorteberg W, Bakke S, Lindegaard K, Boysen M, Nornes H. Cerebral haemodynamics in internal carotid artery trial occlusion. Acta Neurochirurgica. 1997; 139:1066–1073.

30. van Rooij WJ, Sluzewski M, Metz NH, Nijssen PC, Wijnalda D, Rinkel GJ, Tulleken CA. Carotid balloon occlusion for large and giant aneurysms: evaluation of a new test occlusion protocol. Neurosurgery. 2000; 47:116–121. discussion 122.

31. Viñuela F, Fox A, Debrum G, Peerless S, Drake C. Spontaneous carotid cavernous fistulas: clinical, radiological, and therapeutic considerations. Experience with 20 cases. J Neurosurg. 1984; 60:976–984.




 PDF
PDF ePub
ePub Citation
Citation Print
Print





 XML Download
XML Download