Abstract
Purpose
Vitamin D deficiency is common in Crohn disease (CD). The aim of the study was to examine the prevalence of vitamin D deficiency and evaluate the association between vitamin D status and growth outcome in Korean pediatric CD patients.
Methods
In this retrospective study, 17 children younger than 18 years old diagnosed with CD were enrolled and their serum 25-hydroxy vitamin D (25[OH]D) was checked between 2011 and 2015. We categorized the patients into two groups, Group 1 and Group 2. Group 1 included patients with serum 25(OH)D levels below 10 ng/mL, and Group 2 was for patients with a 25(OH)D serum levels between 10 ng/mL and 30 ng/mL. The z-scores for height (Htz), weight (Wtz), and body mass index (BMIz) were measured at baseline, 6 months, and 12 months.
Results
The mean serum 25(OH)D levels of the total 65 CD patients and 17 enrolled patients were 15.64±6.9 ng/mL and 13.1±5.1 ng/mL, respectively. There was no correlation at the beginning of the study between vitamin D level and growth parameters (Htz, Wtz, BMIz) or other variables including laboratory data and Pediatric Crohn Disease Activity Index. The Htz, Wtz, and BMIz in Group 1 showed no significant improvement at 6 months and 12 months follow-up. In Group 2, Wtz and BMIz showed significant improvements sustained until 12 months of follow-up. Htz showed no significant improvement at 6 months but there was significant improvement at 12 months.
Crohn disease (CD) is a chronic inflammatory disease of the gastrointestinal tract. In more recent years though, cases of CD have become more prevalent beyond Western countries, and are observed in increasing frequencies in Asian countries such as Korea [1234].
Significant complications are associated with CD. Of the children diagnosed with CD, 65% to 85% of them show signs of malnutrition and growth retardation. Furthermore, 11% to 37% of these children fail to reach their full potential predicted height in their adulthood [5678].
Traditionally adequate level of vitamin D has been known for its positive impact on bone metabolism. More recent studies have expanded beyond this role, and the broader significance of adequate levels of vitamin D have come to be associated with low risk for cancers, diabetes, cardiovascular disease, autoimmune diseases, and inflammatory bowel diseases (IBD) such as CD [9].
Vitamin D levels are generally divided into three tiers with serum 25-hydroxyvitamin D (25[OH]D) levels of less than 20 ng/mL and between 21 and 29 ng/mL represent vitamin D deficiency and insufficiency, respectively. And, serum 25(OH)D levels between 30 and 100 ng/mL is considered the normal range [101112].
Korea have shown high rates of vitamin D deficiency. According to the Korea National Health and Nutrition Examination Survey (KNHANES V-1), the median 25(OH)D level of Koreans above 10 years old is no more than 20 ng/dL, 10 units lower than the recommended minimum levels of normal vitamin D requirement (30-100 ng/dL) [13].
There is a lack of conclusive studies whether vitamin D levels have effects on pediatric CD in vitamin D deficient regions. This study aims to evaluate the prevalence of vitamin D deficiency in Korean pediatric CD patients. Additionally, keeping in mind that growth retardation is a common complication of pediatric CD, this study also examines whether baseline vitamin D levels has a correlation with the children's growth outcome.
This study reviewed data for 65 patients diagnosed with CD (<18 years old) at the Department of Pediatrics in the Seoul National University Children's Hospital, a tertiary medical center in Seoul, Korea. The serum 25(OH)D levels of these 65 patients were checked from 2011 to 2015. The diagnosis of CD was made based on conventional radiological, histopathological, and endoscopic criteria.
Exclusion criteria are as follows:
(1) Patients who changed the induction treatment method (infliximab, immunomodulatory agent) after checking the 25(OH)D level.
(2) Patients who couldn't visit the hospital at 6 and 12 months after checking the 25(OH)D level.
(3) Children who have a history of small bowel resection. Data were retrospectively obtained from the patient's medical and laboratory records.
Patients' demographic and disease characteristics, such as age, sex, symptoms (abdominal pain, diarrhea, hematochezia, weight loss), extraintestinal manifestations (fever, joint symptoms, oral ulcers), small bowel involvement, and disease activity were collected. Disease activity was measured using the Pediatric Crohn Disease Activity Index (PCDAI); the score indicates: mild disease, 10 to 27.5; moderate disease, 30 to 37.5; and severe disease, 40 to 100 [14]. Small bowel involvement was investigated by imaging studies, such as small bowel series, magnetic resonance enterography, and abdominal computed tomography. Anthropometric data (height, weight, body mass index [BMI]), which were collected at baseline, after 6 months, and after 12 months, were transformed into z-scores for height (Htz), weight (Wtz), and BMI (BMIz) using the 2007 Korean National Growth Chart. Systemic inflammatory markers, including erythrocyte sedimentation rate (ESR), C-reactive protein (CRP), platelet count (PLT), serum albumin (ALB), and hemoglobin (Hb), were collected from patients' laboratory records.
The serum 25(OH)D levels were recorded to estimate vitamin D status. Vitamin D deficiency and insufficiency were defined as levels of serum 25(OH)D of <20 ng/mL and 20-30 ng/mL, respectively. In our study, we define severe vitamin D deficiency and moderate vitamin D deficiency as serum 25(OH)D levels of <10 ng/mL and 10-20 ng/mL, respectively. On the basis of baseline serum 25(OH)D levels, the patients were categorized into Groups 1 and 2. Group 1 included patients with baseline serum 25(OH)D levels of <10 ng/mL, severe vitamin D deficiency. Group 2 included patients with baseline serum25(OH)D levels of 10-30 ng/mL, moderate vitamin D deficiency/insufficiency [151617]. Once a patient was diagnosed as vitamin D deficient, they received oral vitamin D supplements.
All data were analyzed using IBM SPSS Statistics software ver. 20.0 (IBM Co., Armonk, NY, USA). Continuous data of all enrolled patients, such as age and serum 25(OH)D level, are presented as the mean±standard deviation (SD). Categorical data are presented as number (valid percent). Continuous variable in categorized groups, such as anthropometric data (Htz, Wtz, BMIz) and laboratory data (Hb, ESR, CRP, and ALB) are expressed as the mean±SD. Changes in anthropometric data (Htz, Wtz, BMIz) for each group were assessed by the Student paired t-test for normally distributed data and one way ANOVA for non-normally distributed data.
Correlations between variables were checked with the Pearson's correlation coefficient. p-values of <0.05 were considered statistically significant.
This study was approved by the institutional review board at Seoul National University Hospital (IRB no. H 1607-078-776).
A mean serum 25(OH)D level of the 65 CD patients was 15.64±6.9 ng/mL; vitamin D deficiency was found in 81.5% (53/65) and insufficiency in 16.9% (11/65) of patients. Forty-eight patients were excluded: 34 were not available for follow-up at 6 and 12 months, 13 underwent small bowel resection, and 1 changed induction treatment. Therefore, 17 patients were enrolled in the study. The mean age was 13.7 years (29.4% females). The mean 25(OH)D serum level was 13.1±5.1 ng/mL. Vitamin D deficiency and insufficiency were present in 16 patients (94.1%) and 1 patient (5.9%), respectively.
Six patients (35.3%) had severe vitamin D deficiency (Group 1) and 11 patients (64.7%) had moderate vitamin D deficiency/insufficiency (Group 2). The mean serum 25(OH)D level at diagnosis for Group 1 was 8.7±1.4 ng/mL, and the mean level for Group 2 was 15.6±4.7 ng/mL (p<0.001).
There was no significant difference between Group 1 and 2 regarding anthropometric data (Htz, Wtz, BMIz). Compared to Group 1 patients, Group 2 patients had higher PLTs (331.8×103±79.3/µL vs. 508.1×103±192.2/µL, p=0.021). Other laboratory data including Hb, ESR, CRP, and ALB were similar between groups. Moreover, PCDAI and frequency of symptom and extraintestinal manifestations between groups did not show a statistically significant difference (Table 1).
Compared to baseline, all anthropometric data (Htz, Wtz, BMIz) in Group 1 showed no significant improvement at 6 and 12 months' follow-up (Table 2). In the analysis of Group 2, anthropometric data showed significant improvements in Wtz and BMIz sustained throughout the 12 months of follow-up. Htz showed no significant improvement at 6 months, but there was a significant improvement at 12 months (Table 2).
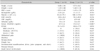
In an effort to evaluate the confounding relationships, we examined the associations between predictors. The Pearson's correlation coefficient found no linear correlation between baseline serum 25(OH)D levels and other variables, including laboratory data (Hb, ESR, CRP, and ALB), anthropometric data (Htz, Wtz, BMIz), and PCDAI (Table 3).
Previous studies reported varying prevalence of hypovitaminosis D in adults with IBD ranging from 16% to 95%, depending on the study. However, relatively few studies focused on the vitamin D status of pediatric IBD patients. A study by Pappa et al. [1819] reported that the prevalence of vitamin D deficiency was 5.8% to 34.6% in pediatric patients with IBD, whereas a recent study found vitamin D deficiency in 48% of children with IBD, although they did take vitamin D supplements [20].
Most current studies evaluate the association of vitamin D status with disease activity index and biochemical parameters. However, most of them were done on adults, and few or no studies have evaluated the association of vitamin D status and growth in pediatric CD patients. The aim of this study was to examine the association of vitamin D status with growth retardation in Korean pediatric patients with CD. In Korea, vitamin D insufficiency is very common in ordinary person and was found in 47.3% of male and 64.5% of female. Also, the prevalence of vitamin D insufficiency in ordinary person is higher in students and young adults than the elderly [13]. This study identified vitamin D deficiency in 81.5% (53/65) and insufficiency in 16.9% (11/65) of our pediatric patients with CD. That is, 98.5% of our pediatric CD patients had hypovitaminosis D. This prevalence in our study was higher than has been reported previously [181920].
Vitamin D is widely known as a key regulator of bone health and in children vitamin D deficiency leads to bone deformities and stunted growth [101112]. More recently, vitamin D has also come to be recognized as an immune regulator and a few studies describe the role of vitamin D in the pathogenesis, clinical outcome, and candidates for adjunctive treatment of CD [21]. Jørgensen et al. [22] showed that vitamin D treatment as a maintenance therapy in CD patients reduced the risk of relapse from 13% to 29%. We could assume that high vitamin D levels are associated with a better clinical outcome. A high vitamin D level would have a positive effect on growth and clinical outcome because growth retardation is mainly secondary to disease activity. Recently, an association between low vitamin D level and higher risk for surgery and hospitalizations for CD [23], and an association between low vitamin D level and loss of response to immunomodulatory treatments were also reported [24].
In our study, Group 2 (moderate vitamin D deficiency/insufficiency) patients showed a significant improvement in Wtz and BMIz after 6 months, which was sustained through to the 12 months' follow-up. Although Htz at 6 months showed no significant improvement, it reached a significant improvement at 12 months. However, Group 1's severely vitamin D deficient patients had no significant improvement of Htz, Wtz, and BMIz during follow-up (6 and 12 months) despite oral vitamin D supplements. For the patients of Group 1, there was no correlation between vitamin D levels and growth parameters (Htz, Wtz, BMIz) or other laboratory variables (Hb, CRP, PLT, ESR, ALB) and PCDAI. The findings indicate that baseline vitamin D status has an effect on growth outcome in pediatric CD. We would like to suggest vitamin D as an adjunctive treatment for pediatric CD patients suffering from growth failure.
It is important to note that the limitations of the current study, these include a small sample size and the retrospective nature of the clinical research process. The patient received oral vitamin D supplements after study entry, but we did not recheck the serum levels and also failed to verify the vitamin D levels after the 12 months of this research.
In conclusion, baseline vitamin D status is a predictor of growth outcome in pediatric CD patients. Future prospective studies of a larger cohort would be required to validate these results. Furthermore, a longer follow-up with vitamin D supplementation will be necessary to determine the appropriate supplemental dose of vitamin D.
Figures and Tables
Table 3
Correlation between Vitamin D Level and Growth Parameter and Other Variables

Vitamin D: serum 25-hydroxyvitamin D, Hb: hemoglobin, PLT: platelet count, CRP: C-reactive protein, ESR: erythrocyte sedimentation rate, ALB: albumin, PCDAI: Paediatric Crohn's Disease Activity Index, Htz: height z-score, Wtz: weight z-score, BMIz: body mass index z-score.
*Statistically significant.
References
1. Vortia E, Kay M, Wyllie R. The role of growth hormone and insulin-like growth factor-1 in Crohn's disease: implications for therapeutic use of human growth hormone in pediatric patients. Curr Opin Pediatr. 2011; 23:545–551.

2. Yang SK, Yun S, Kim JH, Park JY, Kim HY, Kim YH, et al. Epidemiology of inflammatory bowel disease in the Songpa-Kangdong district, Seoul, Korea, 1986-2005: a KASID study. Inflamm Bowel Dis. 2008; 14:542–549.

3. Thia KT, Loftus EV Jr, Sandborn WJ, Yang SK. An update on the epidemiology of inflammatory bowel disease in Asia. Am J Gastroenterol. 2008; 103:3167–3182.

4. Leong RW, Lau JY, Sung JJ. The epidemiology and phenotype of Crohn's disease in the Chinese population. Inflamm Bowel Dis. 2004; 10:646–651.

5. Alhagamhmad MH, Day AS, Lemberg DA, Leach ST. An update of the role of nutritional therapy in the management of Crohn's disease. J Gastroenterol. 2012; 47:872–882.

6. Lee JJ, Escher JC, Shuman MJ, Forbes PW, Delemarre LC, Harr BW, et al. Final adult height of children with inflammatory bowel disease is predicted by parental height and patient minimum height Z-score. Inflamm Bowel Dis. 2010; 16:1669–1677.

7. Shamir R. Nutritional aspects in inflammatory bowel disease. J Pediatr Gastroenterol Nutr. 2009; 48:Suppl 2. S86–S88.

8. Sawczenko A, Ballinger AB, Savage MO, Sanderson IR. Clinical features affecting final adult height in patients with pediatric-onset Crohn's disease. Pediatrics. 2006; 118:124–129.

9. Theodoratou E, Tzoulaki I, Zgaga L, Ioannidis JP. Vitamin D and multiple health outcomes: umbrella review of systematic reviews and meta-analyses of observational studies and randomised trials. BMJ. 2014; 348:g2035.

10. Rosen CJ. Clinical practice. Vitamin D insufficiency. N Engl J Med. 2011; 364:248–254.
11. Holick MF, Binkley NC, Bischoff-Ferrari HA, Gordon CM, Hanley DA, Heaney RP, et al. Evaluation, treatment, and prevention of vitamin D deficiency: an Endocrine Society clinical practice guideline. J Clin Endocrinol Metab. 2011; 96:1911–1930.

13. Choi HS, Oh HJ, Choi H, Choi WH, Kim JG, Kim KM, et al. Vitamin D insufficiency in Korea--a greater threat to younger generation: the Korea National Health and Nutrition Examination Survey (KNHANES) 2008. J Clin Endocrinol Metab. 2011; 96:643–651.

14. Levine A, Griffiths A, Markowitz J, Wilson DC, Turner D, Russell RK, et al. Pediatric modification of the Montreal classification for inflammatory bowel disease: the Paris classification. Inflamm Bowel Dis. 2011; 17:1314–1321.

15. Ulitsky A, Ananthakrishnan AN, Naik A, Skaros S, Zadvornova Y, Binion DG, et al. Vitamin D deficiency in patients with inflammatory bowel disease: association with disease activity and quality of life. JPEN J Parenter Enteral Nutr. 2011; 35:308–316.

16. Tajika M, Matsuura A, Nakamura T, Suzuki T, Sawaki A, Kato T, et al. Risk factors for vitamin D deficiency in patients with Crohn's disease. J Gastroenterol. 2004; 39:527–533.

17. Andreassen H, Rix M, Brot C, Eskildsen P. Regulators of calcium homeostasis and bone mineral density in patients with Crohn's disease. Scand J Gastroenterol. 1998; 33:1087–1093.

18. Pappa HM, Gordon CM, Saslowsky TM, Zholudev A, Horr B, Shih MC, et al. Vitamin D status in children and young adults with inflammatory bowel disease. Pediatrics. 2006; 118:1950–1961.

19. Pappa HM, Langereis EJ, Grand RJ, Gordon CM. Prevalence and risk factors for hypovitaminosis D in young patients with inflammatory bowel disease. J Pediatr Gastroenterol Nutr. 2011; 53:361–364.

20. Laakso S, Valta H, Verkasalo M, Toiviainen-Salo S, Viljakainen H, Mäkitie O. Impaired bone health in inflammatory bowel disease: a case-control study in 80 pediatric patients. Calcif Tissue Int. 2012; 91:121–130.

21. Mora JR, Iwata M, von Andrian UH. Vitamin effects on the immune system: vitamins A and D take centre stage. Nat Rev Immunol. 2008; 8:685–698.

22. Jørgensen SP, Agnholt J, Glerup H, Lyhne S, Villadsen GE, Hvas CL, et al. Clinical trial: vitamin D3 treatment in Crohn's disease-a randomized double-blind placebo-controlled study. Aliment Pharmacol Ther. 2010; 32:377–383.





 PDF
PDF ePub
ePub Citation
Citation Print
Print



 XML Download
XML Download